1. Background
Long-term opioid drug usage results in the development of dependence and tolerance that reduces their beneficial use and creates severe health and social concerns (1). In numerous forms of drug addiction, negative and positive reinforcement include two main elements. Positive strengthening of euphoric consequences leads to leads to pursuing drug, while negative reinforcement of withdrawal signs happens following an interruption of opioid taking (2-4). Opiate dependence is a multifaceted occurrence that engages several areas of the brain (2, 5-8).
The neuropeptides orexin A and orexin B are synthesized, particularly in the hypothalamus, and act on target neurons throughout the central nervous system via two G-protein-coupled receptors, orexin 1 (OXR1) and 2 receptors (OXR2) (9, 10). In the previous studies, it has been revealed that orexinergic neurons of the dorsomedial hypothalamus and perifornical area are activated by foot shock stimulus and are complicated in the negative reinforcement of withdrawal symptoms (4, 11). Nucleus raphe magnus that is a thermoregulatory center (12, 13) has high densities of not only opiate receptors but also orexin receptors (14). Orexinergic neurons have projections to the visual cortex that send information to the lateral geniculate nucleus (LGN). Accordingly, LGN and many other thalamic nuclei have orexin receptors (15, 16). Orexin is involved in long-term potentiation that leads to learning and memory (17, 18). Another study described that the injection of orexin in the nucleus tractus solitarius that is a regulator of the cardiovascular system, causes blood pressure increase (19-21). Recently, compelling studies have demonstrated a novel and significant role for the orexin neuronal system in reward processing and addiction. Morphine-conditioned animals had greater Fos-activated orexin neurons than non-conditioned ones (22). Orexin is involved in reward processing and addiction in the nucleus accumbens, ventral tegmental area, and the locus coeruleus that receives glutamatergic afferents mainly from paragiganto cellular nucleus (14, 23-26).
Although several pieces of evidence demonstrated an effect of orexins in arousal and preservation of the waking conditions (27), additional evidence supports a significant and particular role in reward procedures and drug abuse as well (28). Injection of the OXR1 antagonist SB-334867 systemically or into the ventral tegmental area prevented the acquirement of cocaine sensitization (29). Furthermore, orexin is implicated in cue-induced drug-craving and motivation to take cocaine when a high effort is essential to acquire the drug, but not in the principal reinforcing properties of cocaine itself (30).
In the previous studies, hematological factors in heroin and opium-dependent subjects were investigated (31, 32). Nevertheless, in heroin-dependent groups, several alterations occur in immune function and blood lymphocytes (33). Similarly, other studies displayed that opioid intake may exacerbate along with the progress of infectious diseases (31). On the other hand, it was revealed that inhibition of OXR1 reduced the development of morphine tolerance and physical dependence in rats (34). Totally, previous studies concluded that SB-334867 was used for the decrement of morphine dependence and withdrawal behaviors (35).
2. Objectives
Therefore, we aimed to investigate the effect of OXR1antagonist injection on hematologic factors in morphine-dependent rats.
3. Patients and Methods
3.1. Drugs
Morphine sulfate (Temad, Tehran, Iran) was dissolved in a volume of 10 mg per 1ml physiological saline then was administered subcutaneously. The selective OXR1 antagonist SB-334867 (Tocris, Bristol, UK) was dissolved in 2% dimethyl sulfoxide, 10% 2-hydroxypropyl-β-cyclodextrin in sterile water purchased from Sigma-Aldrich, Germany.
3.2. Animals
Male Wistar rats were accommodated in Plexiglas breeding cages in groups of four for each cage with woodchip bedding and ad libitum access to food and water. Animals were housed in a colony room at ambient temperature and on 12-h light/dark cycles (the light period started at 7 a.m.). Efforts were made to diminish animal distress and reduce the number of animals that were used. All experiments were done in accordance with the ethical guidelines set by the “Ethical Committee of Iran University of Medical Sciences”, which are according to the “NIH Guide for the Care and Use of Laboratory Animals”.
3.3. Induction of Morphine Dependence
To induce morphine dependence, morphine was injected (10 mg/Kg, 1ml, subcutaneous [s.c.]) twice a day with an interval of 12 hours for seven days (36).
3.3.1. Assessment of Hematologic Factors
To detect the level of hematological parameters, the subsequent steps were carried out: 2 mL fresh venous blood was collected in test tubes comprising specific EDTA anticoagulant. Afterward, the subsequent tests were conducted on the samples by the usage of Counter Sysmex:
1. Complete blood cell count (CBC) for red and white blood cell
2. Hemoglobin (HGB) level
3. Hematocrit percentage (HCT)
4. Cell indices, including mean corpuscular hemoglobin (MCH), mean corpuscular volume (MCV), and mean corpuscular hemoglobin concentration (MCHC)
5. Differential leukocyte count (neutrophils)
6. Blood platelets (PLT)
3.4. Experimental Groups
Rats were divided into five different experimental groups as the following: Group 1 (sham), rats were naïve without any injection (n = 6); Group 2 (control), rats received SB-334867 vehicle (2% dimethyl sulfoxide (DMSO), 10% 2-hydropropyl-β-cyclodextrin in sterile water) P1-30 (37) and then DMSO+ cyclodextrin +Saline (morphine vehicle) P31-P37 (n = 8) ; Group 3 (M); animals (P31) received morphine administration (10 mg/kg, 1 mL, s.c.) for seven days twice a day (n = 8); Group 4 (SB), animals received SB-334867 (20 mg/kg, Intraperitoneal [i.p.]) (4) daily from P1-P37 (n = 6). Group 5 (SB+M), animals (n = 6) received SB-334867 (20 mg/Kg, i.p.) (4) daily from P1-P30 and then before each morphine injection for seven days.
3.5. Data Analysis
Data are expressed as mean ± SEM and were analyzed using unpaired two-tailed Student t-test and one-way analysis of variance (ANOVA) for the comparison of two or more groups, respectively. The defined level of statistical significance was P < 0.05.
4. Results
No significant differences were observed in the WBC, MCHC, HCT, and platelet levels between the naïve group and vehicle-treated rats (Table 1). The comparison between naïve and vehicle-treated rats displayed that the injection process has not any effect on the level of mentioned hematologic factors.
| Groups/Hem. Factor | Naïve | SB-334867 Vehicle | Difference |
|---|---|---|---|
| WBC (× 103/µL) | 4.5 ± 1.15 | 3.73 ± 0.39 | NS |
| HCT (%) | 35.50 ± 4.20 | 30.75 ± 1.72 | NS |
| MCHC (%) | 29.85 ± 0.05 | 31.13 ± 0.33 | NS |
| Platelet (× 103/µL) | 360.7 ± 16.23 | 383.6 ± 5.87 | NS |
Abbreviations: NS, non-significant
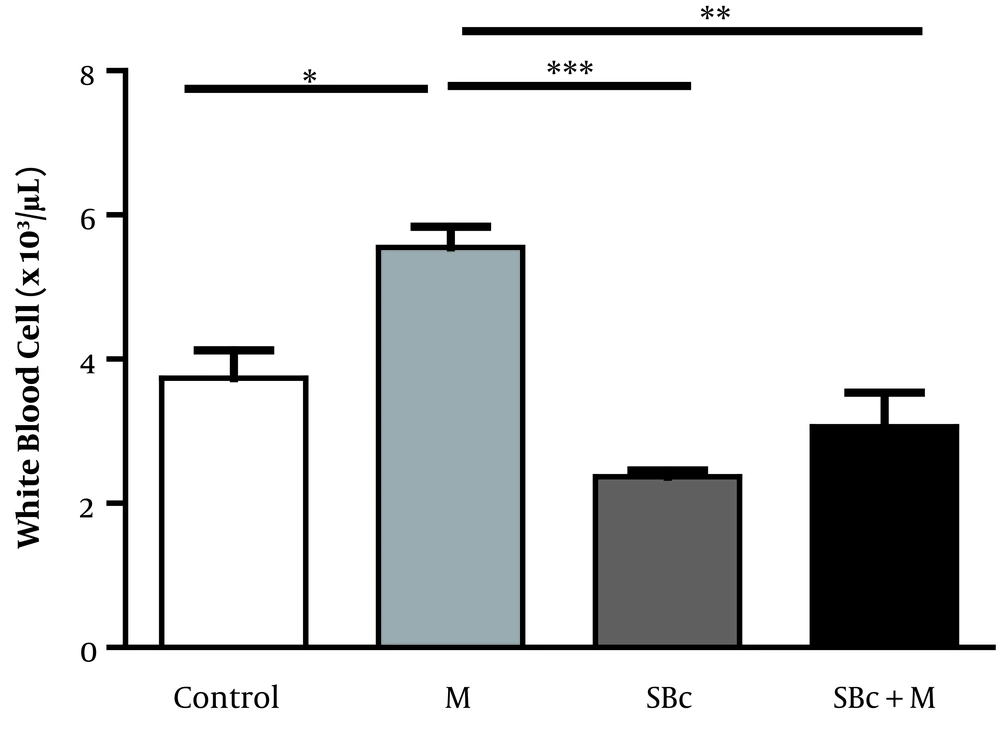
4.1. The Effects of Morphine Addiction and Orexin Antagonist on WBC Counts
The results of our study indicated that the mean number of total WBC in the morphine-dependent rats (5.550 ± 0.289 × 103 cells/µL) increased compared to the control group (3.733 ± 0.393 × 103 cells/µL) while SB-334867 could decrease the WBC in morphine-dependent rats (3.067 ± 0.470 × 103 cells/µL) compared to control rats (P < 0.01, Figure 1).
The lymphocyte numbers in morphine-dependent rats noticeably decreased compared to the control group (2726 ± 99 and 1589 ± 155 cells/µL, P < 0.001, unpaired t-test), while the number of eosinophils in morphine-dependent rats (2726 ± 96 cells/µL) showed no difference in comparison to the control group (2726 ± 95 cells/µL, unpaired t-test). SB-334867 failed to change the number of lymphocytes and eosinophil in the morphine-dependent rats compared to the control group.
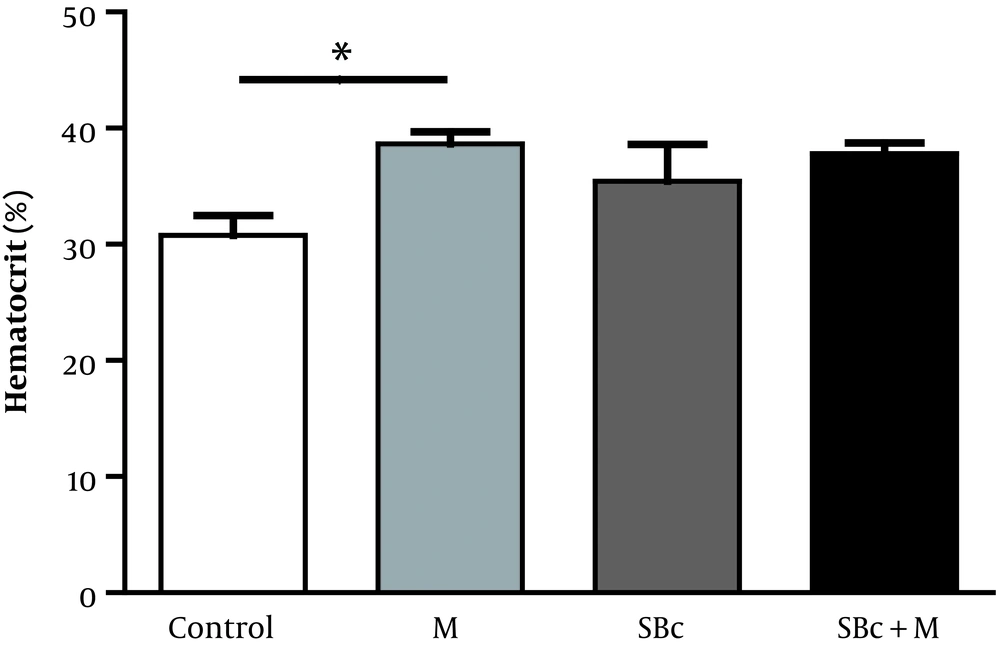
4.2. The Effects of Morphine Addiction and Orexin Antagonist on Hematocrit Level
The results of the current study indicated that the mean number of HCT in the morphine-addicted rats (36.8 ± 2.78%) increased (P < 0.05) compared to the control group (30.7 ± 3.45%), while SB-334867 failed to change the HCT in morphine-dependent rats (37.8 ± 1.79%) compared to the morphine-addicted rats (Figure 2).
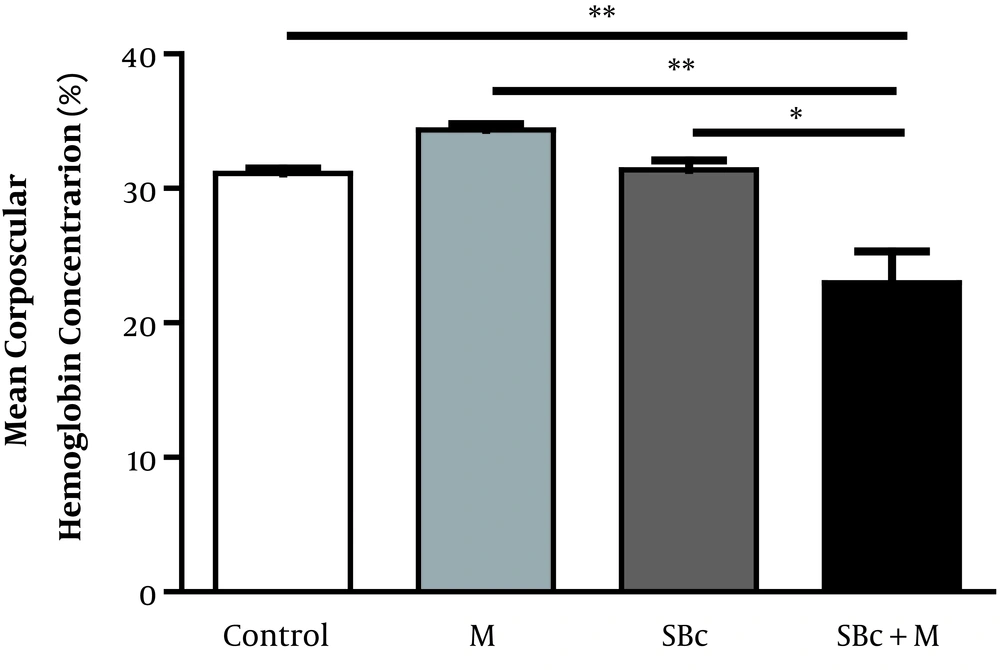
4.3. The Effects of Morphine Addiction and Orexin Antagonist on MCHC Counts
The results of our study indicated that the MCHC in the morphine-addicted rats (34.35 ± 0.45%) increased compared to the control group (31.13 ± 0.33%), while SB-334867 decreased the WBC in morphine-dependent rats (22.98 ± 2.35%) compared to the control rats (P < 0.01, Figure 3).
The effect of SB-334867 on the mean corpuscular hemoglobin concentration (MCHC) of morphine-dependent rats (M). The graph displays the MCHC in morphine-dependent rats who received SB-334867 (orexin antagonist) (SBc+M) compared to control and morphine-injected rats (M). Data are shown as mean ± SEM (*P < 0.05, **P < 0.01).
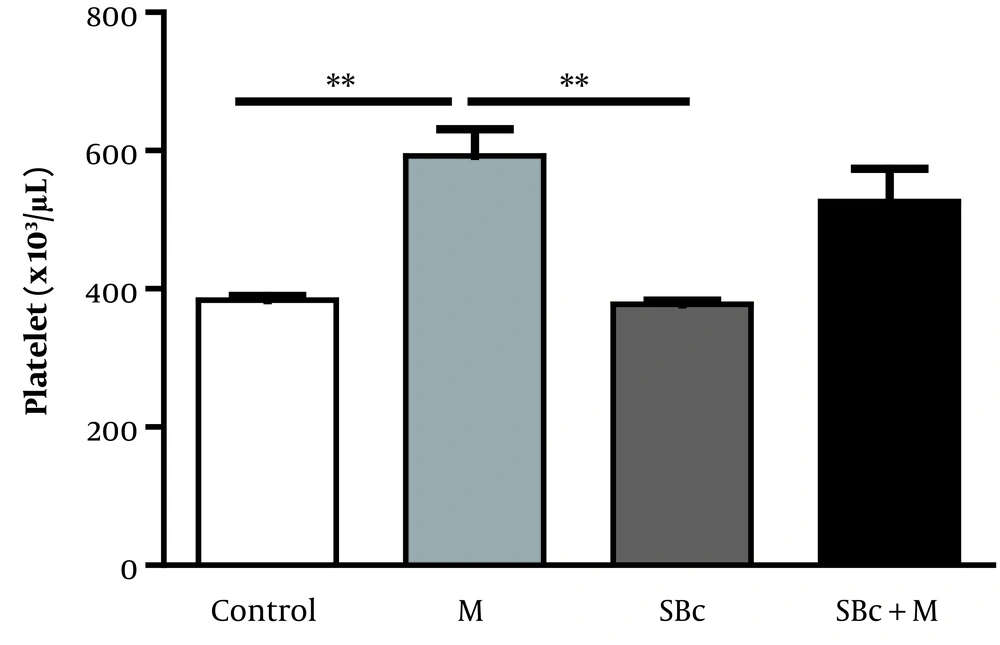
4.4. The Effects of Morphine Addiction and Orexin Antagonist on Platelet Counts
The platelet count was increased by morphine (592.2 ± 38.970 × 103 cells/µL) compared to the control group (383.600 ± 5.87 × 103 cells/µL) (P < 0.01). The injection of SB-334867 in morphine-dependent rats decreased, and so returned platelet counts to the control level (Figure 4).
No significant differences were observed in RBC levels between the morphine-addicted group (5.56 ± 0.07 × 103 cells/µL) and control (5.27 ± 0.28 × 103 cells/µL) and between SB-treated (5.72 ± 0.4 × 103 cells/µL) and SBc+M (6.098 ± 0.40 ×103 cells/µL). There were no significant differences in the mean hemoglobin between the morphine-addicted group (11.7 ± 1.24 g/dL) and the control (10.3 ± 1.07g/dL) as well as between the SB-treated (11.3 ± 1.18g/dL) and SBc+M (12.5 ± 1.96 g/dL) rats. No significant differences were observed in the MCV level between the morphine-addicted (63.6 ± 1.07 fL) and control groups (65.2 ± 2.81 fL) as well as between the SB-treated (63.7 ± 1.67 fL) and the SBc+M (65.8 ± 1.97 fL) rats (Table 2).
| Index | SB Vehicle | M | SBc | SBc + M |
|---|---|---|---|---|
| RBC (×103/ µL) | 5.27 ± 0.28 | 5.56 ± 0.07 | 5.72 ± 0.4 | 6.098 ± 0.40 |
| HGB (g/dL) | 10.3 ± 1.07 | 11.7 ± 1.24 | 11.3 ± 1.18 | 12.5 ± 1.96 |
| MCV (fL) | 65.2 ± 2.81 | 63.6 ± 1.07 | 63.7 ± 1.67 | 65.8 ± 1.97 |
| MCH (pgm) | 21.5 ± 2.49 | 22.6 ± 2.08 | 19.7 ± 0.61 | 20.5 ± 0. 45 |
| RDW (%) | 23.0 ± 5.42 | 20.6 ± 0.28 | 22.4 ± 2.08 | 18.4 ± 0.36 |
Abbreviations: HGB, hemoglobin; MCH, mean cell hemoglobin; MCV, mean corpuscular volume; RBC, red blood cell; RDW, red cell distribution width.
aData are shown as mean ± SEM.
The MCH level (22.6 ± 2.08 pg) did not show any difference in the morphine-dependent group in comparison to the control group (21.5 ± 2.49 pg). In addition, SB-treated (19.7 ± 0.61 pg) and SBc+M (20.5 ± 0. 45 pg) had not any significant difference (Table 2).
The percentage of the red blood cell distribution width (RDW) in the morphine-dependent group (20.6 ± 0.28) did not reveal any significant difference compared to the control group (23.0 ± 5.42). In addition, there was no significant difference in the RDW level between the SB-treated rats (22.4 ± 2.08) and SBc+M-treated ones (18.4 ± 0.36) (Table 2).
5. Discussion
Along with numerous problems derived from narcotic drugs, the results of the current research unraveled the intense outcomes of morphine on the total and differential counts of peripheral WBC and platelets, as well as HCT and MCHC levels. Furthermore, the current results demonstrated that orexin antagonist was involved in some changes caused by morphine, and hence, it might suppress several depraved effects of morphine on the above-mentioned hematologic factors.
5.1. The Effect of Orexin Antagonist on WBC in Morphine-Dependent Rats
In this study, it has been revealed that morphine addiction shows an intense impact on the mean total WBC count. In previous studies, it has been revealed that morphine, as a component of opium, prompts catecholamine release, which is identified to raise the leukocyte count (38). Rising of the peripheral blood leukocyte count also might be augmented by direct injury of epithelial and endothelial surfaces and/or alterations in cytokine levels (especially interleukin-6 [IL-6]) produced by constituents of opium (39).
It would be mentioned that the hemopoiesis is controlled by a multipart network of cytokines like colony-stimulating factors. It has been established that opium or some of its derivatives may play a role in the secretion of cytokines such as IL-2, IL-4, IL-5, IL-10, IFN-γ, and TGF-β (40), thereby influencing the production of WBC.
It is previously demonstrated that in heroin-dependent people without any malnutrition, blood T cell count was augmented (41, 42). In addition, there are several reports suggesting that the treatment of heroin and morphine would strengthen some parameters of the immune system. In this regard, it was revealed that the creation of several cytokines was augmented a few minutes after morphine injection in mice (32). Moreover, evidence displayed that the monocyte count was greater than the normal level in heroin-addicted persons (43, 44). Morphine is known as the agonist of μ opioids receptors and the central and the active metabolite of heroin. The possible mechanism of the morphine effect might be the regulation of the immune system either directly through mu opiate receptors situated on the immune cells, or indirectly via a central way with the mu receptors in the central nervous system.
In a previous study on the morphine-dependent dogs, it was demonstrated that morphine could not change the number of WBCs (45). In another study on the mouse whose, bone marrow cells implanted with morphine pellets for 72 h exhibited a 65% reduction in their response to macrophage colony-stimulating factor (M-CSF). Nevertheless, they used morphine for 72h, while in the current study, morphine injection was done at a longer time. Thus, different duration of injection may have various effects (46).
Although the result of total white blood cell count disclosed a significant increment in morphine-dependent rats, the number of lymphocytes reduced by morphine. In the previous studies in both male and female rats, the number of lymphocytes was considerably smaller in addicted-diabetic animals than diabetic non-addicted ones (47). Moreover, a diminished number of lymphocytes has been reported in addicted dogs (48). The current results exemplify that opium addiction has intense adverse effects on lymphocytes counts. The mentioned outcomes may be ascribed to the apoptotic aspects of opium or its derivatives on lymphocytes (49, 50).
The current results indicated that the injection of SB-334867 significantly decreased WBC counts in the morphine-dependent rats. It was also revealed that OXR1 and 2 were expressed on human CD34+ blood stem and progenitor cells (51). The central downstream signaling pathways of the orexin receptors comprise Ca2+-dependent signaling accompanying with activation of mitogen-activated protein kinase (MAPK) and extracellular signal-related kinase 1/2 (ERK1/2) pathways (52).
Evidence revealed that ERK (1/2) was activated at a maximum of 3 hours of subsequent incubation with orexin-A (53). In addition, stimulation with orexin-A resulted in a significantly higher ratio of early pluripotent hematopoietic progenitor (CFU-GEMM) colonies (54). Thus, the above evidence indicates that WBC reduction by SB-334867 might be due to inhibition of orexin activation effect on ERK1/2 or Colony-forming unit-granulocyte, erythroid, macrophage, megakaryocyte (CFU-GEMM). Therefore, SB-334867 can prevent WBC increment induced by morphine.
5.2. The Effects of Orexin Antagonist on Hematocrit Level and MCHC in Morphine-Dependent Rats
In the current study, morphine did not change MCHC significantly. It is consistent with previous studies, demonstrating that six-month heroin addiction did not make key alterations in erythrocyte parameters, for instance, RBC count and HGB levels (55). Correspondingly, it was revealed that the blood HGB content did not change in morphine-dependent dogs (56). In comparison with the present study, it was revealed that blood HGB was augmented in people who used heroin. Nevertheless, in methadone-addicted people, the amount of HGB reduced to its normal level (31). In another study, it was revealed that HGB concentration decreased in morphine-dependent people, but they found that HCT increased by morphine (31). Thus, the increment of both HGB and HCT in morphine-dependent subjects may lead to MCHC stability by morphine. The current results, however, indicated that the injection of SB-334867 significantly decreased MCHC in morphine-dependent rats. The previous studies demonstrated that stimulation with orexin A and B led to a significant decline of erythroid precursors burst forming unit erythrocyte (BFU-E) and colony-forming unit erythrocyte (CFU-E) (54). Hence, HCT reduction by orexin could be due to the diminishing of these erythroid precursors.
In addition, it was discovered that orexin-A usage enhanced the expression of nuclear factor erythroid-derived 2 related factor 2 and antioxidant response element luciferase activity, resulting in the generation of the cytoprotective enzyme heme oxygenase-1 (HO-1) (57). This could catabolize heme to biliverdin, carbon monoxide, and free iron (58). Consequently, it may be proposed that orexin decreased HGB by HO-1 activating, and as a result, SB-334867 could increase the HGB.
Although in this study, HGB and HCT increased by SB-334867 in morphine-dependent rats, the results were not statically significant. Hence, it may propose that the HCT increment was higher than that of HGB, which could decrease the MCHC level indeed.
5.3. The Effect of Orexin Antagonist on Platelet Number in Morphine-Dependent Rats
Our results demonstrated that morphine injection could increase platelet counts. Enhanced platelet activity amplifies significantly the risk of arterial thrombotic diseases, including stroke, peripheral ischemia, and myocardial infarction (59).
Our results demonstrated that SB-334867 injection could return platelet counts to the control level in morphine-dependent rats; thus may prevent numerous cardiovascular disorders, which could be due to morphine dependence. Previous studies have been revealed that orexins augmented the excitability and synchronization of rat sympathetic preganglionic neurons. Centrally administered orexins display cardiovascular effects, comprising a rise of blood pressure and heart rate (60). Therefore, totally, it is concluded that SB-334867 can prevent cardiovascular diseases directly by orexin receptor antagonization or indirectly by the prevention of morphine effect on platelet counts.
The current study, however, indicated that SB-334867 did not alter the results of the rest of hematologic factors, including MCV, MCH, RBC, RDW, HGB in morphine-dependent rats. This might be due to the great level of deviation detected in these factors.
5.4. Conclusion
SB-334867 reduced MCHC, WBC, and platelet counts that were increased in morphine-dependent rats. Therefore, totally it may be concluded that blockade of OXR1 may improve morphine-induced changes in hematologic factors of morphine-dependent rats.