1. Background
Methicillin-resistant Staphylococcus aureus (MRSA), one of the oldest multidrug-resistant (MDR) gram-positive bacteria, was discovered in 1960. It is one of the leading pathogens responsible for healthcare-associated infections (HAIs), including hospital-acquired pneumonia (HAP) and ventilator-associated pneumonia (VAP) that are consistently increasing over the last few decades worldwide (1, 2). VAP can be defined as pneumonia developed within 48 - 72 hours or thereafter as a consequence of endotracheal intubation commonly characterized by the presence of one or more pathogenic organisms in the respiratory tract specimens with signs of systemic infections including fever, elevated white blood cell count, and elevated infection markers (3). VAP is developed in 9% - 27% of all mechanically ventilated patients in intensive care units (ICUs) worldwide (3), and ventilator-associated MRSA pneumonia (VAMP) is a serious infection in intubated patients with a high rate of hospital-mortality and morbidity (1).
The prevalence rate of MRSA-associated infections is remarkable all over the world, and 77.5% of VAP cases in Asia and Latin-America are caused by MRSA (4). According to the statement of the German national nosocomial infection surveillance system (KISS), in German ICUs, out of 16,000 VAP cases, 37% are VAMP per year, indicating that about 7,500 VAMP cases occur in all European ICUs annually (5). VAP with MRSA is difficult-to-treat because MRSA strains are generally found fully-resistant to multiple antibiotics. The most troublesome issue is that MRSA infections, including VAMP, are solely unresponsive to first-line-antibiotics, and this situation demands the use of reserve or last-line antibiotics such as linezolid, vancomycin, teicoplanin, and daptomycin as the first-line therapy (6).
Linezolid, as the first antibiotic of the oxazolidinone class approved by the Food and Drug Administration (FDA) of the United States (US) in 2000, is one of the most potential antibiotics with multiple goods in vitro and in vivo experiences about MRSA (2, 6). Similarly, the glycopeptides antibiotic teicoplanin, widely used in European countries, is mostly intended for the treatment of moderate to severe gram-positive bacterial infections, including MRSA.7 Numerous studies found good therapeutic outcomes of both linezolid and teicoplanin, individually used in VAMP (1, 2). Adverse drug reactions (ADRs) in critically ill patients are unwanted incidences, causing additional physical or clinical complications in the patients or aggravating their current disease states (7). The development of ADRs with linezolid or teicoplanin during the course of therapy in critically ill patients is a serious issue concerning drug safety (2, 8, 9). The therapeutic potentiality and frequency of developing ADRs may result in a safer drug with a better therapeutic effect, and the same perception implicates users to choose a better one from the two antibiotics, i.e., linezolid or teicoplanin, in VAMP treatment.
2. Objectives
The prime objective of this study was to compare the clinical outcomes and associated drug safety (in terms of adverse drug reaction) of the linezolid and teicoplanin therapy in the treatment of ventilator-associated MRSA pneumonia.
3. Methods
3.1. Study Design and Sample
This single-center retrospective study was conducted in the intensive care unit (ICU) of a tertiary care hospital in Dhaka, Bangladesh. Patients developing VAMP in the ICU from February 1, 2018, to January 31, 2019, were considered in this study. During this period, among the 731 admitted South Asian patients in the ICU, 238 patients were intubated with mechanical ventilation. Out of the 238 intubated patients, 98 adult patients (age > 18 years) developed VAMP. For VAMP treatment, either linezolid (LZD) [dose: 600 mg intravenously, every 12 h (no renal dosage adjustment done)] or teicoplanin (TPN) [loading dose: 400 mg intravenously, every 12 h for three doses; maintenance dose: 400 mg every 24 hours (renal dosage adjustments were performed according to the hospital’s standard antibiotic dosing guideline)] was administered to the patients (N = 98) in two separate groups, i.e. 42 and 56 patients were assigned to the LZD and TPN groups, respectively. The total duration of the prescribed linezolid and teicoplanin therapy was intended to be 14 days considering the disease severity of the VAP patients. The samples were randomly distributed into two distinct groups (LZD and TPN groups) using the ‘lottery sampling’ method. All the patient data for this study were collected from the hospital’s “online patient data archive,” and patient-wise prescribed medication histories were collected from the “online indent record service” in the pharmacy of the hospital. In this study, patients in both groups (LZD and TPN) were on mechanical ventilation support, when the endotracheal aspirate (ETA) cultural sensitivity (CS) report confirmed MRSA pneumonia. Following the confirmation of lung infection with MRSA (through the ETA CS report), linezolid or teicoplanin was prescribed by a physician responsible for MRSA pneumonia treatment based on the hospital’s antimicrobial stewardship guideline. The CS testing methodology (micro-broth dilution) and equipment (BD Phoenix™ M50 automated microbiology system) of the microbiology lab were validated according to the standards of the Clinical and Laboratory Standards Institute (CLSI), the United States. All the antibiotic therapy-related clinical outcome data of patients in both groups were compared to each other. All the data of ADRs were collected from the clinical pharmacists’ daily ADR monitoring record book.
3.2. Sample Inclusion and Exclusion Criteria
The inclusion criteria were receiving LZD or TPN for VAMP treatment; no previous history of allergy to LZD and TPN; MRSA (in the ETA CS report) sensitive to LZD and, or TPN; actual/adjusted body weight above 40 kg; no previous history of intubation; and not smoking.
History of any gram-positive bacterial infection including MRSA pneumonia, and associated treatment history with/without LZD or TPN within the last three weeks of the mentioned study period; patients with the acute physiology and chronic health evaluation (APACHE II) score above 30 during ICU admission; patients developing additional gram-positive or -negative bacterial infection (s) at the same time; the death of patients before completing two weeks of LZD or TPN therapy; patients shifted outside ICU or discharged from the hospital during LZD or TPN therapy; patients with a history of mild to severe acute/chronic liver diseases, hematological disorders, asthma, or chronic obstructive pulmonary diseases (COPD); and patients with serum creatinine level above 2.5 mg/dL or history of chronic kidney disease or end-stage renal disease were considered as the exclusion criteria.
3.3. Definitions
MRSA is considered multidrug-resistant when the MRSA isolates are entirely resistant to common penicillins, cephalosporins, fluoroquinolones, macrolides, and carbapenems (6). Clinical success was assessed based on the complete microbial eradication rate in the first CS report (performed after completing six days of the antibiotic therapy). LZD and TPN therapy-associated ADRs detected during the therapy were collected from the clinical pharmacists’ patient-wise ‘online intervention record system’ and enlisted in each group (LZD or TPN). Thrombocytopenia can be defined here as a platelet count < 150,000 cells/mm3 or a 50% decreased platelet count below the baseline value. Nephrotoxicity can be defined here as a 50% increased serum creatinine level above the baseline value. The platelet count and the serum creatinine level measured within 24 h before confirming VAMP was considered as the baseline value in this study.
In this study, the safety of LZD/TPN therapy was evaluated by estimating the discontinuation rate of the LZD or TPN therapy before the 14th day of the therapy due to the development of LZD- or TPN-associated adverse reactions.
3.4. Statistical Analysis
Data were analyzed with SPSS version 22.0 statistical software (SPSS, Chicago, IL, US). All the tests were two-sided. Fisher’s exact test and Pearson’s chi-square test were performed to compare the categorical variables, whereas Student’s t-test was used to compare the continuous variables. Values were expressed as mean ± SD (standard deviation). A P value ≤ 0.05 was considered statistically significant. The Square Hospital Ethical Committee granted ethical approval for this study on January 14, 2018.
4. Results
The mean age of patients in the LZD and TPN groups was 54.7 and 53.1 (P > 0.05), respectively (Table 1). The primary infection marker C-reactive protein (CRP) of each patient in both groups was determined with a mean value (± SD) for the LZD (211.5 ± 103.2) and TPN (176±105.7) groups before starting the group-wise antibiotic therapy (P = 0.078). For further confirmation of the severity of infection, the procalcitonin level was assessed for every patient in both groups before going to CS, and higher procalcitonin levels than the reference standard in all the patients manifested significant bacterial infection. Table 1 shows that the levels are statistically significant (P = 0.007). The mean (±SD) white blood cell count of the patients in the LZD and TPN groups was 16.9 (SD = ± 4.6) and 18.4 (SD = ± 4.9), respectively. The renal function condition of patients in both groups was highly similar, and the mean (± SD) serum creatinine level was 1.6 ± 0.5 and 1.5 ± 0.5 for the LZD and TPN groups, respectively (P > 0.05) (Table 1). The mean (± SD) platelet count of patients in the LZD and TPN groups was 270.8 ± 63.93 and 279.9 ± 57.36, respectively, with a P value of > 0.05 (Table 1). The acute physiology and chronic health evaluation (APACHE) II score of patients in both groups was <30 measured during admission to ICU, and the mean ± SD of the LZD and TPN groups was 13.8 ± 2.8 and 17.5 ± 4.2, respectively (P < 0.05) (Table 1).
| Characteristics | Variables | P Value | |
|---|---|---|---|
| LZD Group (N = 42) | TPN Group (N = 56) | ||
| Age (year) | |||
| Mean ± SD | 54.7 ± 17.3 | 53.1 ± 11.5 | 0.558 |
| Range (min - max) | 22 - 73 | 21 - 69 | |
| Gender | |||
| Male | 28 | 35 | 0.813 |
| Female | 14 | 21 | |
| C-reactive protein (< 10.0 mg/mL) | |||
| Mean ± SD | 211.5 ± 103.2 | 176 ± 105.7 | 0.078 |
| Range (min - max) | 11.7 - 390.4 | 12.3 - 377.2 | |
| Procalcitonin (< 0.1 ng/mL) | |||
| Mean ± SD | 41.5 ± 56.7 | 21.2 ± 35.2 | 0.007 |
| Range (min - max) | 2.1 - 224.4 | 1.6 - 182.2 | |
| White blood cell (4 - 11 K/µL) | |||
| Mean ± SD | 16.9 ± 4.6 | 18.4 ± 4.9 | 0.283 |
| Range (min - max) | 12.4 - 31.4 | 11 - 32.6 | |
| Platelet (150 - 450 K/µL) | 0.785 | ||
| Mean ± SD | 270.8 ± 63.93 | 279.9 ± 57.36 | |
| Range (min - max) | 159 - 452 | 165 - 412 | |
| Serum creatinine (0.8 - 1.4 mg/dL) | |||
| Mean ± SD | 1.6 ± 0.5 | 1.5 ± 0.5 | 0.756 |
| Range (min - max) | 0.6 - 2.3 | 0.6 - 2.5 | |
| APACHE II Score (0 - 30) | 0.005 | ||
| Mean ± SD | 13.8 ± 2.8 | 17.5 ± 4.2 | |
| Range (min - max) | 10 - 20 | 10 - 24 | |
Abbreviations: APACHE, acute physiology and chronic health evaluation
The linezolid and teicoplanin therapy in the LZD and TPN groups was respectively continued for 14 days from the date of confirmation of VAMP. In the LZD group, 80.9% (34, n = 42) of the patients completed the full duration of the therapy with a microbial eradication rate of 97% (33, n = 34). However, in the TPN group, 94.6% (53, n = 56) of patients completed teicoplanin therapy with a microbial eradication rate of 94.3% (50, n = 53), which was significantly higher than that in the LZD group [the full linezolid course was completed in 80.9% (34, n = 42) of the patients; the microbial eradication rate was 97% (33, n = 34)] (Table 2).
| Outcome | LZD Group, No. (%) | TPN Group, No. (%) | P Value |
|---|---|---|---|
| 14-day therapy completion | 34 (80.9) (n = 42) | 53 (94.6) (n = 56) | 0.034 |
| Therapy discontinuation before first CS-review | 8 (19) (n = 42) | 3 (5.3) (n = 56) | 0.034 |
| Microbial eradication | 33 (97) (n = 34) | 50 (94.3) (n = 53) | 0.543 |
| Extension of the duration of treatment (> 14 days) | 8 (19) (n = 42) | 3 (5.3) (n = 56) | 0.034 |
| Total ADR recorded | 8 (19) (n = 42) | 3 (5.3) (n = 56) | 0.034 |
| ADR detected < 48 h of therapy | 3 (37.5) (n = 8) | 1 (33.3) (n = 3) | 0.185 |
| ADR detected < 96 h of therapy | 5 (62.5) (n = 8) | 2 (66.6) (n = 3) | 0.241 |
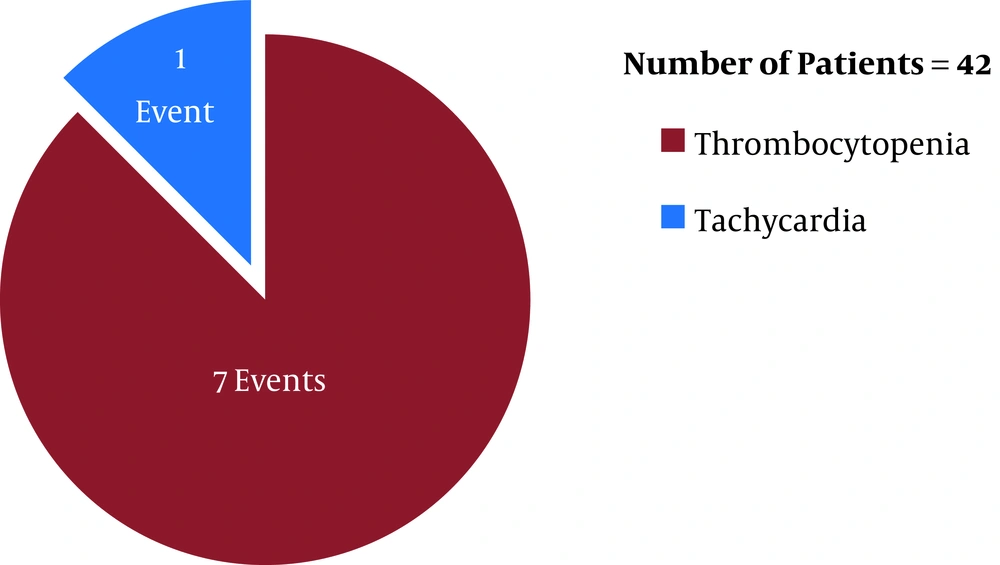
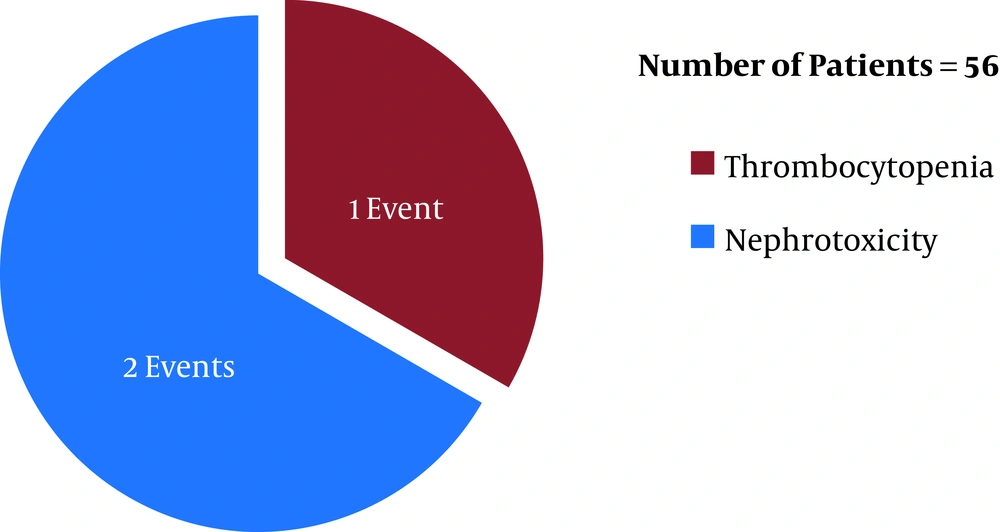
Before the first ETA CS review (after six days of the initiation of the antibiotic therapies), linezolid (in LZD group) was discontinued in eight patients (19%, n = 42) whereas teicoplanin (in TPN group) was discontinued in three patients (5.3%, n = 56) (P = 0.034) due to linezolid- and teicoplanin-induced adverse reactions [eight (19%, n = 42) and three (5.3%, n = 56) adverse events occurred in the LZD and TPN groups, respectively]. In respect of time, in the LZD group, 37.5% (three, n = 8) (one tachycardia and two thrombocytopenia events) and 62.5% (five, n = 8) (five thrombocytopenia events) of ADR incidences occurred within 48 hours and 96 hours post-antibiotic therapy initiation, respectively, with linezolid (Figure 1). In contrast, in the TPN group, 33.3% (1, n = 3) (one nephrotoxicity event) and 66.6% (two, n = 3) (one thrombocytopenia and one nephrotoxicity event) of ADR incidences occurred within 48 hours and 96 hours post-antibiotic therapy initiation, respectively, with teicoplanin (Figure 2) (P > 0.05). Out of eight ADRs in the LZD group, seven were thrombocytopenia, and one was tachycardia (Figure 1) (where the patient was on norepinephrine as an ionotropic agent). On the other hand, three ADRs were found in the TPN group, where two adverse events were the increased serum creatinine level (nephrotoxicity), and one adverse event was thrombocytopenia (Figure 2).
All the ADRs in both groups were managed with therapeutic interventions in required cases. Due to the sudden discontinuation of the current antibiotics (linezolid in the LZD group and teicoplanin in the TPN group) as a result of ADRs with the corresponding antibiotics and incorporation of new antibiotics, the total duration of the MRSA-treatment was extended (> 14-day of antibiotic therapy) in 19% (eight, n = 42) and 5.3% (three, n = 56) (P = 0.034) of patients in the LZD and TPN groups, respectively.
5. Discussion
The results of this study indicated that patients with VAMP treated with linezolid (the LZD group) had a significantly higher MRSA eradication rate (97%) compared to patients treated with teicoplanin (the TPN group) (94.3%). In a double-blind, randomized, multicenter study on ICU-patients, linezolid had a higher MRSA clearance rate (51.1%) than teicoplanin (18.6%), linezolid showed superior clinical success (78.9%) than teicoplanin (72.8%), and two MRSA isolates exhibited reduced susceptibility to teicoplanin (2). An IMPACT-HAP study evaluated the therapeutic effectiveness of linezolid compared to vancomycin in VAP patients and found that linezolid possessed 85% clinical success, whereas vancomycin showed 69% (1). Another prospective, double-blind trial showed an 11% higher therapeutic success rate in favor of linezolid compared to its counterpart in VAMP (10). It has been hypothesized that linezolid penetrates lung tissues well enough, resulting in better clinical outcomes in patients with VAMP (11).
The increasing trend of drug-resistant virulent strains of Staphylococcus aureus and its associated difficult-to-treat infections, including VAMP, in ICU patients, has already been considered as an emerging threat for the global public health. The FDA of the United States has approved linezolid and vancomycin for VAMP treatment, and some European countries have additionally incorporated teicoplanin and quinupristin/dalforpristin to their practice (12). Worldwide, VAMP treatment is highly challenging because of few last-line potential antibiotics, mostly limited to linezolid, teicoplanin, and vancomycin, and also, due to the prolongation of antibiotic therapy (14 to 21 days of therapy) (13). The most alarming issue is that several studies have reported the resistance of MRSA strains to these reserve antibiotics (14-16). In the South Asian countries, this situation has come up as a result of irrational dosing of these antibiotics, lack of the CS review in recommended intervals, and inadequate therapeutic drug monitoring systems (1).
The serum drug concentration is a significant concern for yielding optimal therapeutic effectiveness of teicoplanin, and suboptimal teicoplanin trough level may exhibit reduced clinical success (8). However, 12 hourly 600 mg adult dosing of linezolid maintains a sufficient minimum inhibitory concentration (MIC) to eradicate MRSA, and no dosage adjustment is required in any degree of liver or kidney impairment or in any severity of infections (17); it also does not need any extra therapeutic trough level monitoring (2). In an in-vitro experiment, the oxacillin resistant MRSA strain (ATCC 43300) was susceptible to teicoplanin at a MIC of 0.5 mg/L and for resistant mutants, this level could be 2 to ≥ 16 mg/L (8). The variable drug levels required to kill MDR-MRSA isolates are difficult to safely observe the reference standard, where no therapeutic drug level monitoring facility for teicoplanin is available, like our study setup.
Linezolid binds to the 50S ribosomal subunits of gram-positive bacteria. Then, 70S functional initiation complex formation is blocked, and finally, bacterial protein synthesis is inhibited, which ultimately kills the bacteria (18). In addition to the extremely high clinical success rate of linezolid in VAMP treatment, several studies reported linezolid-induced multiple serious ADRs, including thrombocytopenia, anemia, and tachycardia (19-21). A study showed that VAP patients treated with linezolid developed thrombocytopenia (17.8%) with a hospital-mortality rate of 9.9% (1). Linezolid-induced thrombocytopenia follows a concentration-dependent mechanism (20), and the frequency of this ADR increases in patients with renal impairment (19). Gerson et al. elucidated that linezolid-induced thrombocytopenia resulted from the toxicity-induced suppression of bone marrow and hematopoietic cells (22). However, later on, Bernstein et al. demonstrated that specific antibody-mediated autoimmune reaction was the main reason behind this reaction, and they totally refused the previous concept of linezolid-induced thrombocytopenia’s mechanism (23). Our study did not consider the relationship between renal impairment and thrombocytopenia in patients treated with linezolid, but thrombocytopenia frequently occurred in the LZD group’s patients.
Linezolid possesses weak reversible monoamine oxidase (MAO)-A and B inhibitory characteristics (24), and these enzymes are responsible for the metabolism of epinephrine, norepinephrine, and serotonin (25). A single 600 mg dose of linezolid yields a serum drug level ~18 µg/mL, which is sufficient to inhibit MAO-A and MAO-B potentially. When given with the nonselective MAO inhibitor and the serotonin reuptake inhibitor (SSRI), this may result in serotonin toxicity or serotonin syndrome, including tachycardia (24). Here, one patient in the LZD group developed linezolid-induced tachycardia, which may be the result of serotonin toxicity while the patient was on norepinephrine. Based on the patient’s clinical condition, linezolid therapy was withdrawn immediately, and the reaction was subsided.
Teicoplanin is a reserve antibiotic in the potential antibiotic line for the treatment of MDR-MRSA infections, including VAMP (7). Few studies have reported teicoplanin-induced neutropenia, hemolytic anemia (26-28), and thrombocytopenia (29-31). By using the indirect platelet immunofluorescence test method, a study demonstrated that teicoplanin-dependent antibodies were produced in patients treated with teicoplanin at regular dosages with significant target-specificity to glycoprotein IIb/IIIa (GPIIb/IIIa) available on platelet cells, and rarely caused thrombocytopenia when the antibodies were clinically significant (32). A systemic review and meta-analysis found less frequent teicoplanin-induced nephrotoxicity (elevated serum creatinine level from the baseline value) (RR, 0.44; 95% CI, 0.32 to 0.61), and teicoplanin-induced acute interstitial nephritis leading to irreversible nephrotoxicity was first reported in 1992 (33). In our study, we experienced two teicoplanin-induced nephrotoxicity cases, where the creatinine level returned to its baseline value within 48 hours after the discontinuation of teicoplanin.
In this study, linezolid showed a relatively higher clinical success rate in the treatment of MDR-MRSA VAP compared to teicoplanin. However, only 80.9% of linezolid therapy successfully finished its intended course of therapy, while teicoplanin showed a higher course completion record (94.6%). As a result, the extended duration of treatment (19%) was required in patients treated with linezolid compared to patients treated with teicoplanin (5.3%). The reason was the development of unwanted ADRs with linezolid and teicoplanin. Higher incidences of linezolid-induced ADRs (19%) ultimately resulted in further discontinuation of linezolid therapy before reviewing the first CS report, more shifting to suitable alternate antibiotic therapy, and prolongation of the course of antibiotics (> 14 days) for treating MDR-MRSA-associated VAP in the LZD group than in the teicoplanin group. The increased rate of linezolid-induced adverse events while using in VAMP treatment with significant clinical outcomes places questions to clinicians regarding the therapeutic drug safety of linezolid in VAMP, which may exacerbate complications in critically ill VAP patients, prolong hospitalization time, and increase the treatment cost. In contrast, teicoplanin was found with better drug safety due to its less number of adverse events in VAMP treatment with a highly similar rate of MRSA eradication to linezolid (linezolid: 97% and teicoplanin: 94.3%), resulting in significant clinical outcomes. Thus, teicoplanin may be a better therapeutic option for MRSA-associated VAP treatment in critically ill patients, given its higher drug safety and promising clinical outcomes compared to linezolid.
A single-center study with a small sample size in both groups was the main limitation of this study. In addition, no mortality rate calculation among the groups, no data on the further complication of the disease states in the patients, and lack of data on the resistance profile of MRSA-caused infections were among other limitations of the study.
5.1. Conclusion
MRSA infections are always difficult-to-treat for clinicians, and linezolid has been the drug-of-choice for the last few decades in the treatment of VAP associated with MRSA worldwide. In this study, frequent adverse events induced with linezolid further complicated the disease states in mechanically ventilated patients. However, teicoplanin showed similar therapeutic efficacy to linezolid with remarkably lower adverse events in critically ill VAMP patients. Therefore, given the better therapeutic drug safety and clinical outcomes of teicoplanin, it may be superior to linezolid in ventilator-associated MRSA pneumonia treatment.