1. Background
Malaria is a mosquito-borne zoonotic infectious disease caused by protozoan parasites, which invade the red blood cells. The human symptoms include fever, fatigue, headache, nausea, shaking chills, convulsion, coma, abdominal, and muscle pain. The symptoms begin 10-15 days after mosquito bites, which, if left untreated, will recur in successive months (1).
Malaria is more prevalent in tropical and semi-tropical areas, including Sub-Saharan Africa, Asia, and Latin America (2-4). 214 million malaria cases were observed universally, resulting in 434,000 deaths, of which 90% occurred in Africa (5). Iran is as an endemic site for malaria with 519 cases in 2013, from which 80% were vivax (6, 7). Iran showed a 75% reduction in malaria infection between 2000 and 2013; however, the incidence rate of malaria was 10 to 100 cases per 100,000 inhabitants. 90% of cases were seen in south-eastern parts, including Sistan and Baluchistan, Hormozgan, Kerman, and Fars provinces. Precipitation and immigratebility of the residential areas are from the most important causes for high prevalence in these parts (8, 9).
Some diseases are recurrent to a specific space and time. The usual way of assessing these variables was to categorize one dimension and analyze it in another dimension or vice-versa. However, modern technologies such as ArcGIS and SaTScan, with which permutation scan modeling was used, have enabled us to investigate the space and time features of these variables at the same time. This work was the first methodology to check the space-time trait of malaria simultaneously and detect the space-time clusters in Fars province-Iran during 2011 and 2015 (10-13).
2. Methods
Time-series data, including 357 malaria cases recorded from 19 cities, were used retrospectively to detect the space-time clusters of malaria in Fars province-Iran during 2011 and 2015. Since it was a retrospective study, no consent form was applied; however, all the ethical steps including data collection and analysis as well as reporting the results were performed in accordance with the standards approved by Ethics Committee of Ministry of Health, Treatment, and Medical Education under ethics number of IR.SUMS.REC.1396.S755.
Median ± IQR (interquartile range), frequency, and relative frequency were used to describe the quantitative and qualitative data, respectively. Kolmogorov-Smirnov, chi-square, Kruskal-Wallis, and space-time permutation scan modeling were used to analyze the data. SPSS V. 22, ITSM 2002, ArcGIS10, and SaTScan9.4.4 software tools were used. The significance level was considered 0.05.
The space-time trait of a variable was evaluated using the space-time permutation model introduced by Kulldorff using permutation scan statistics (10). Finally, only clusters were reported with no geographical overlap. The same analysis was conducted to detect clusters within large clusters.
3. Results
3.1. Descriptive Statistics
357 cases recorded from 19 cities of Fars province during 2011 and 2015 were involved in the study. The minimum number of cases was 0 in Abade, Bavanat, and Fasa, and the largest numbers were in Shiraz 63.9%, Jahrom 11.2%, Larestan 7.8%, and Kazeroun 5%, respectively.
3.2. Time Trend
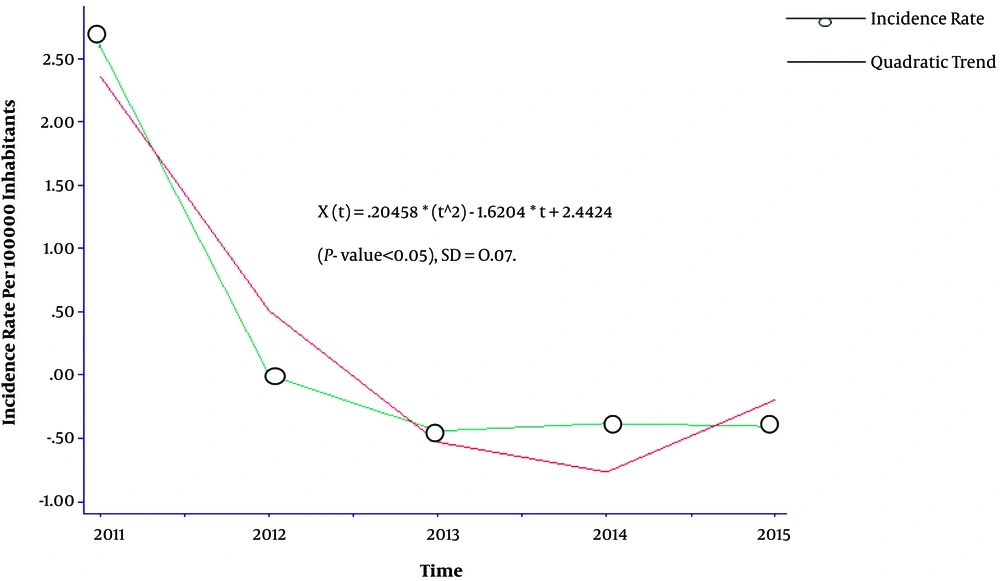
A quadratic trend was seen in the annual rates of malaria cases during 2011 and 2015 in Figure 1.
Malaria cases were significantly different over 2011 - 2015 years (P < 0.05). The difference was due to the differences observed in 2011 with other years (P value < 0.05 for all).
3.2.1. Monthly Time Trend and Geographical Distribution
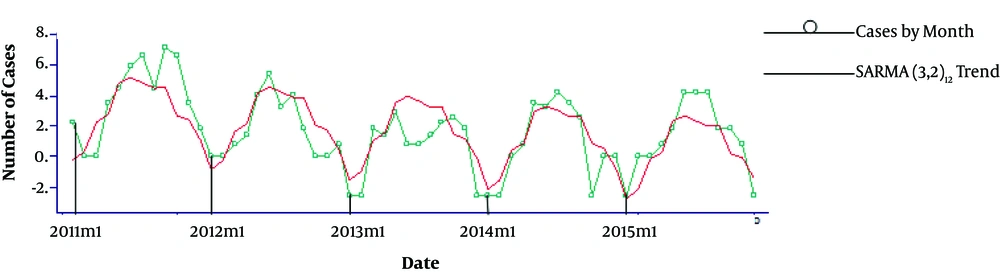
Figure 2 shows the monthly trend of malaria cases from January 2011 to February 2015.
Seasonal Auto-Regressive Moving Average, SARMA (3, 2)12, model was fitted to explain the trend.
The results indicated significant differences among malaria cases in 12 months of the year (P < 0.05) in Table 1. In addition, malaria cases were different in 19 cities of Fars province (P < 0.05) in Table 2.
| January | February | March | April | May | June | July | August | September | October | November | December | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| January | 0.006 | 0.009 | 0.037 | 0.037 | ||||||||
| February | 0.042 | 0.004 | 0.005 | 0.025 | 0.025 | |||||||
| March | 0.01 | 0.013 | ||||||||||
| April | 0.037 | 0.047 | ||||||||||
| May | 0.042 | |||||||||||
| June | 0.006 | 0.004 | 0.01 | 0.037 | 0.029 | 0.007 | ||||||
| July | 0.009 | 0.05 | 0.013 | 0.047 | 0.037 | |||||||
| August | 0.037 | 0.025 | 0.042 | |||||||||
| September | 0.037 | 0.025 | 0.042 | |||||||||
| October | ||||||||||||
| November | 0.029 | 0.037 | ||||||||||
| December | 0.007 | 0.042 | 0.042 |
aValues are statistically significant at 0.05 level.
| Arsenjan | Stahban | Eghlid | Abade | Bavanat | Jahrom | Safashahr | Darab | Sepidan | Shiraz | Farashband | Fasa | Firoozabad | Kazeroun | Larestan | Lamerd | Marvdasht | Mamasani | Neireez | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arsenjan | 0.006 | 0.001 | 0.025 | ||||||||||||||||
| Stahban | 0.004 | 0.001 | 0.013 | ||||||||||||||||
| Eghlid | 0.004 | 0.001 | 0.013 | ||||||||||||||||
| Abade | 0.002 | 0.001 | 0.007 | ||||||||||||||||
| Bavanat | 0.002 | 0.001 | 0.007 | ||||||||||||||||
| Jahrom | 0.006 | 0.004 | 0.004 | 0.002 | 0.002 | 0.002 | 0.004 | 0.002 | 0.002 | 0.01 | 0.043 | 0.006 | 0.006 | ||||||
| Safashahr | 0.002 | 0.001 | 0.007 | ||||||||||||||||
| Darab | 0.001 | ||||||||||||||||||
| Sepidan | 0.004 | 0.001 | 0.013 | ||||||||||||||||
| Shiraz | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.002 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | |
| Farashband | 0.001 | ||||||||||||||||||
| Fasa | 0.002 | 0.001 | 0.007 | ||||||||||||||||
| Firoozabad | 0.001 | ||||||||||||||||||
| Kazeroon | 0.001 | ||||||||||||||||||
| Larestan | 0.025 | 0.013 | 0.013 | 0.007 | 0.007 | 0.007 | 0.013 | 0.001 | 0.007 | 0.046 | 0.025 | 0.025 | |||||||
| Lamerd | 0.01 | 0.001 | 0.046 | ||||||||||||||||
| Marvdasht | 0.043 | 0.001 | |||||||||||||||||
| Mamasani | 0.006 | 0.001 | 0.025 | ||||||||||||||||
| Neireez | 0.006 | 0.001 | 0.025 |
aValues are statistically significant at 0.05 level.
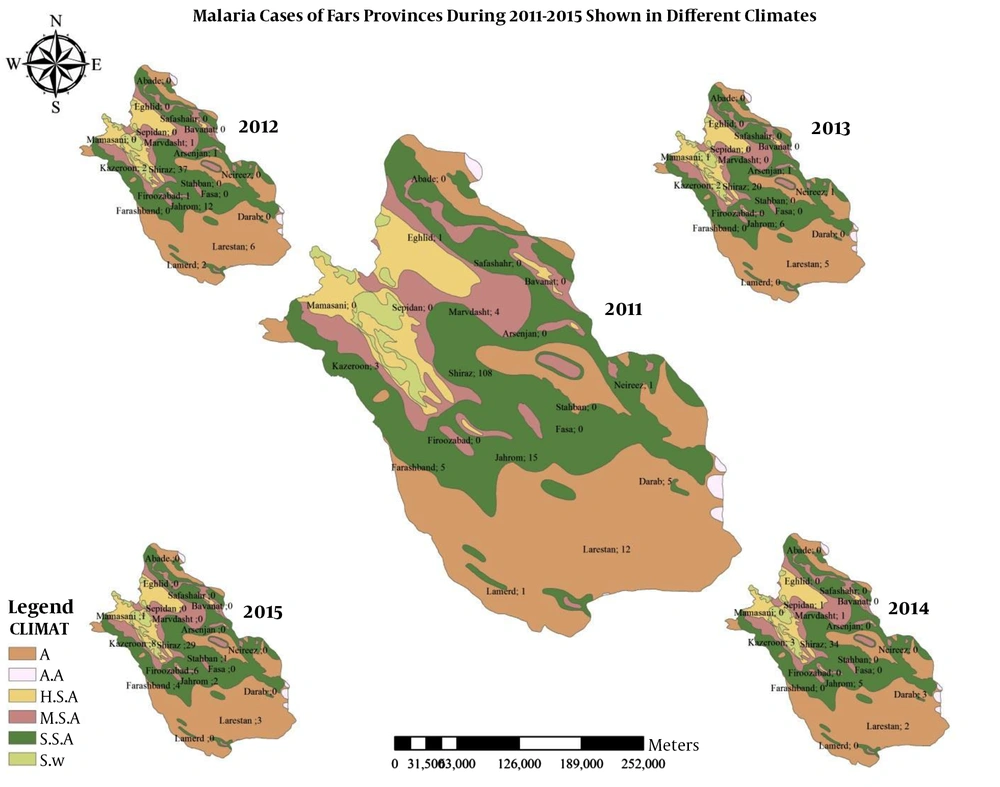
Figure 3 shows the geographical distribution of malaria cases by six different climates of humidity.
3.3. Space-Time Permutation Analysis
In this study, 5 space-time clusters were detected, of which the MLC was statistically significant (P < 0.05). MLC contained 1.7% (6/357) of the total cases, and the other four non-significant clusters contained 10.9% (39/357) of the total cases across the study period. Table 3 shows the results of the permutation space-time analysis.
| Cluster | Location(s) | Radius(km) | Observed/Expected | Time Window | Test Statistics | P-Valuea |
|---|---|---|---|---|---|---|
| Cluster 1 | Firoozabad | 0 | 26 | 1/7/2015 to 31/7/2015 | 13.71 | < 0.0001 |
| Cluster 2 | Kazeroon | 0 | 5.41 | 1/8/2015 to 30/11/2015 | 5.27 | 0.2389 |
| Cluster 3 | Darab | 0 | 26.44 | 1/3/2014 to 30/4/2014 | 4.63 | 0.47 |
| Cluster 4 | Lamerd, Larestan, Jahrom | 136.2 | 1.82 | 1/4/2012 to 31/8/2013 | 4.57 | 0.48 |
| Cluster 5 | Arsenjan, Marvdasht | 46.29 | 12.75 | 1/11/2012 to 31/3/2013 | 3.25 | 0.96 |
aStatistical significance was evaluated using Monte Carlo hypothesis testing.
3.3.1. The Most Likely Cluster (MLC)
The MLC occurred in Firoozabad and contained almost 50% (6/12) of all cases during 1/7/2015 and 31/7/2015. The other 50% of malaria cases occurred in Shiraz [2/12 (16.7%)], Larestan [2/12 (16.7%)], Esfahan [1/12 (8.3%)], and Farashband [1/12 (8.3%)] in July 2015.
3.3.2. Secondary Clusters (SCs)
The four SCs encompassed 10.9% (39/357) of the total cases during the study period. SC1 included Kazeroun, which had 100% (6/6) of the cases during 1/8/2015 and 30/11/2015. In this SC, 66.6% (4/6) of the cases occurred in August, 16.6% (1/6) in September, 16.6% (1/6) in November, and 0% in October (0/6).
SC2 had 100% (2/2) of the cases during 1/3/2014-30/4/2014. Among these cases, 50% were in March, and the other 50% were in April.
SC3 composed 96.6% (29/30) of the cases from 1/4/2012 to 31/8/2013. In this SC, Lamerd, Larestan, and Jahrom had 6.6% (2/30), 33.3% (10/30), and 56.6% (17/30) of all the cases, respectively. Indeed, the largest number of cases over 1/4/2012-31/8/2013 were in May [23.3% (7/30)], June [20% (6/30)], April [13.3% (4/30)], August [13.3% (4/30)], September [10%(3/30)], March[6.6% (2/30)], July[6.6% (2/30)], and December [6.6% (2/30)]. However, no cases were detected in October, November, January, and February.
SC4 had 100% (2/2) of all cases on 1/11/2012-31/3/2013. It encompassed Arsenjan and Marvdasht. Arsenjan and Marvdasht had 50% (1/2) of the total cases in that period. In addition, 50% (1/2) of the cases occurred in July, and the other 50% (1/2) occurred in May.
3.3.3. Subcluster Analysis
It was also interesting to determine whether or not there were any significant clusters within the large clusters. In doing so, the sub-cluster analysis was conducted, showing no statistically significant space-time clusters (P value > 0.05). Table 4 shows the results of the sub-cluster analysis.
| Cluster | Location | Radius (Km) | Start Date | End Date | Test Statistic | Observed/Expected | P-Valuea |
|---|---|---|---|---|---|---|---|
| Cluster 1 | Lamerd | 0 | 1/6/2012 | 31/12/2012 | 1.27 | 3.94 | 0.61 |
| Larestan | 0 | 1/5/2011 | 30/9/2011 | 1.02 | 1.58 | 0.89 | |
| Cluster 2 | Arsenjan | 0 | 1/3/2012 | 31/3/2013 | 0.83 | 2.67 | 0.18 |
aStatistical significance was evaluated using Monte Carlo hypothesis testing.
4. Discussion
The detected space-time clusters showed that malaria followed a space-time feature in the area during 2011 and 2015. 5 clusters were found of which the MLC in Firoozabad as the canonical site for malaria was the only significant cluster. It included 50% of all cases during 1/7/2015 and 31/7/2015. MLC contained almost 1.7% of all cases over the study period. In the current study, the purely temporal analysis revealed an outbreak from June to September (from 2004 to 2006), which was in concordance with the MLC time frame (June (2017)).
Also, the detected time frame of MLC from April 2004 to November (2007) was in agreement with the transmission period of malaria in Fars province (14).
In a study in China, the purely temporal analysis resulted in three clusters happening from March to August (in 2005), from May to October (in 2006), and from March to April (2007). These periods encompassed the transmission period of malaria mainly from April to November. In addition, space-time clustering in the current study detected three clusters, including MLC occurring in 1/1/2005–31/5/2007, one SCs in 1/2/2005–28/2/2007, and another SCs in 1/1/2005–31/1/2005. All these periods covered the transmission time of malaria and proved the efficacy of this methodology on malaria (15).
In a previous study done on cutaneous leishmaniasis (CL), a disease with a very high prevalence in Fars province resulted in discovering space-time clusters in the area. it showed that permutation scan modeling was efficient enough in detecting outbreaks of infectious diseases in the area (11-13).
Unlike our quadratic trend of malaria during 2011 and 2015 in Fars province, some studies showed a decreasing trend over time, especially in vivax malaria (15-17).
Clinical and statistical preferences have always been controversial; permutation scan statistics results in statistically significant and non-significant clusters. Non-significant clusters are clinically canons of infection. Methodologically, it is a drawback in permutation scan modeling. As a solution, Population Attributable Risk (PAR) could help to decide between clinical and statistical significant clusters. PAR is a value between 0 and 1, and the closer this number is to one, the more likely it is to be a real cluster (15). Another limitation was that the surveillance system in Iran was passive. For example, many patients did not go to the health centers, and consequences were not registered in the health system; this leads to underestimating the disease prevalence. Finally, there was a variety of meteorological factors in Fars province, such as precipitation and wind velocity, which were not included in the study. The sero-epidemiological traits of reservoirs and agents and hosts’ genetic features were not considered as well.
Although the data were old, it was the first methodology evaluating space and time traits of malaria in the south of Iran. Mathematically, it was shown that malaria did not follow the uniform distribution over time and space in the area, and space- time clusters could function as an early warning system to the health system. In other words, the findings could be useful in organizing the priorities of facilities in high-risk areas.