1. Background
Since the emergence and escalation of Coronavirus 2019 (COVID-19) to pandemic status, there has been an urgency to identify management options that could treat or at least contain the disease. COVID-19, a viral illness caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (a beta coronavirus) was identified in Wuhan, China in Dec 2019 (1, 2). Initially, antivirals such as combinations of lopinavir-ritonavir, antibiotics such as azithromycin, and anti-malarial agents like chloroquine and hydroxychloroquine with potential anti-viral properties were studied (3-5). Though modest benefits were noted with these treatment options, adverse effects often limited use (4, 5). Favipiravir, ribavirin and interferon are other antivirals that are currently in the clinical trial phase. While the efficacy of ribavirin and interferon remain unclear, Favipiravir showed encouraging initial results and was approved by China for treatment of SARS-CoV-2 infections (2, 3, 5). Remdesivir, a broad-spectrum antiviral that inhibits ribonucleic acid (RNA)-dependent RNA polymerases showed promising results (6). Studies have shown faster viral clearance and recovery time with improved mortality benefit especially in non-critically ill COVID patients (7-10). Remdesivir is currently the only Food and Drug Administration (FDA) approved drug for SARS-CoV-2.
As awareness of the immunopathology associated with SARS-CoV-2 and deleterious effects of cytokine storm evolved, a targeted approach to curbing immune response appeared to be favorable (11, 12). Immunological agents like interleukin (IL)-6 inhibitors such as tocilizumab and siltuximab, interleukins, tumor necrosis factor alpha and corticosteroids were tested. Multiple studies have shown varying results with tocilizumab, though the majority did show improvement in oxygen requirements and clinical improvement (13-18). Steroids showed no mortality benefit. However, the recent randomized evaluation of COVID-19 therapy (RECOVERY) trial by Horby et al. (19) revealed improved outcomes with using the selective glucocorticoid dexamethasone in patients requiring supplemental oxygen.
Despite multiple treatment options, no report of a combination therapy with either remdesivir and IL-6 inhibitors, IL-6 inhibitors and steroids or a combination of all the above was found as of July 11, 2020.
2. Objectives
The objectives of our report was to assess whether a multi-pronged approach to tackling SARS-CoV-2 had an impact on decreasing the need for mechanical ventilation, identify some of the complexities involved in these combination treatment regimens, and suggest that additional studies that examine the sequential use of drugs in the management of these acutely ill patients are needed.
3. Methods
3.1. Study Design, Population, Setting and Data Collection
This retrospective study was conducted at a single tertiary medical center. Six hospitalized patients between ages 50 to 77 years with confirmed SARS-CoV-2 formed the study group. SARS-CoV-2 status was confirmed by reverse transcriptase polymerase chain reaction (RT-PCR) assay of nasopharyngeal swab specimens.
3.2. IRB Waiver
As only anonymized (deidentified) data was included in the study, informed consent was waived.
3.3. Data Collection
Data was gathered through the electronic medical records of the institution. Information obtained included demographic data, comorbidities, presenting clinical signs or symptoms, laboratory and radiological findings along with medications provided.
3.4. Specimen Collection and Testing
COVID status was confirmed by Centers for Disease Control and Prevention (CDC) approved real-time reverse transcription polymerase chain reaction (rRT-PCR) diagnostic panel. RT-PCR uses nucleic acid amplification technology to quantitatively detect and diagnose SARS-CoV-2 from nasopharyngeal samples.
3.5. Inclusion Criteria
Eligible patients included in the study were confirmed SARS-CoV-2 positive patients with active infection including fever, chills, shortness of breath and elevated inflammatory indices [c-reactive protein (CRP), lactate dehydrogenase (LDH), ferritin, D-dimer and low procalcitonin] with chest X-ray showing bilateral hazy infiltrates and use of supplemental oxygen. At time of initiation of treatment, all six patients required at least 3 liters (L) nasal cannula or more of supplemental oxygenation.
3.6. Exclusion Criteria
A negative COVID test result, presence of renal failure, hepatic failure patients on mechanical ventilation or those who were discharged or demised within 24 hours were not included in the study.
3.7. Study Definitions
Six patients with similar demographics and comorbidities were treated with a combination of treatments that included antibiotics, remdesivir, steroids, convalescent plasma and tocilizumab. The end point of this study was to assess whether a combination of medical treatments decreased the chances of the subject requiring invasive mechanical ventilation.
4. Results
4.1. Demographic and Clinical Characteristics of the Patients
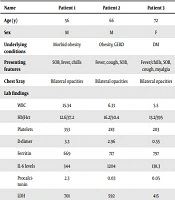
In this study, six patients who tested positive for SARS-COV-2 were followed. Demographics and clinical features (signs and symptoms) of the patients can be seen in Table 1. The patients ranged from 50 to 77 years with the mean age of 64. The study population was predominantly Hispanic. Given their age, most patients had underlying comorbidities with diabetes, hypertension and obesity being the most predominant.
| Name | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 |
|---|---|---|---|---|---|---|
| Age (y) | 56 | 66 | 72 | 50 | 64 | 77 |
| Sex | M | M | F | M | F | M |
| Underlying conditions | Morbid obesity | Obesity, GERD | DM | HTN, DM | DM, HLD | DM, HTN |
| Presenting features | SOB, fever, chills | Fever, cough, SOB, | Fever/chills, SOB, cough, myalgia | Fever, myalgia, HA, SOB, cough | SOB, fever, cough | SOB, fever, chills, cough |
| Chest Xray | Bilateral opacities | Bilateral opacities | Bilateral opacities | Bilateral opacities | Bilateral opacities | Bilateral opacities |
| Lab findings | ||||||
| WBC | 15.34 | 6.33 | 5.5 | 10.82 | 12.17 | 16.9 |
| Hb/Hct | 12.6/37.2 | 16.2/50.4 | 13.2/395 | 10.2/35.1 | 14.9/45.1 | 15.7/48.6 |
| Platelets | 353 | 283 | 203 | 80 | 326 | 223 |
| D-dimer | 3.3 | 2.96 | 0.55 | >20 | 2.59 | 12.57 |
| Ferritin | 669 | 717 | 797 | 1280 | 544 | 475 |
| IL-6 levels | 344 | 1204 | 138.3 | 543 | 153.7 | 798 |
| Procalcitonin | 2.3 | 0.03 | 0.05 | 0.56 | 0.13 | 0.09 |
| LDH | 701 | 592 | 415 | 674 | 544 | 608 |
| Antibiotics | Ceftriaxone, Azithromycin | Ceftriaxone, Azithromycin, Cefepime | Ceftriaxone, Azithromycin | Pip/tazo, Azithromycin | Ceftriaxone, Azithromycin, Cefepime | Ceftriaxone, Azithromycin |
| Steroids | + | + | + | + | + | + |
| Remdesivir | + | + | + | + | + | + |
| IL-6 inhibitor | + | + | + | + | + | + |
| Convalescent plasma | no | + | No | no | + | + |
| Mechanical ventilation | IMV | IMV | No | no | IMV | IMV |
Abbreviations: M, male; F, female; SOB, shortness of breath; HA, headache; Hb, hemoglobin; Hct, hematocrit; DM, diabetes; HTN, hypertension; HLD, hyperlipidemia; GERD, gastroesophageal reflux disease; Pip/tazo, piperacillin/tazobactam; IMV, invasive mechanical ventilation.
4.2. Laboratory and Radiological Findings
At admission, elevated white blood cells were seen in 50% (3/6) of the study population and lymphopenia was seen in 67% (4/6). Analysis of inflammatory markers showed elevation of LDH, ferritin and D-dimer in all six (100%) of the patients. The mean LDH value was 589 and the median D-dimer level was 6.995. Procalcitonin levels were noted to be elevated in 34% of the patients, the mean value of 0.53. Chest X-rays showed bilateral infiltrates in all six (100%) patients (Table 1).
4.3. Management
All six subjects were given a combination of antibiotics (cefepime, ceftriaxone, azithromycin and piperacillin/tazobactam), remdesivir, IL-6 inhibitors (tocilizumab), steroids and convalescent plasma, in no order of preference.
4.4. Outcomes
Adjuvant therapy with remdesivir (anti-viral), tocilizumab (IL-6 inhibitor), convalescent plasma, steroids and antibiotics were provided in all six subjects. All patients received remdesivir for 5 - 10 days, tocilizumab for a total of 4 doses each across 2 days, methyl prednisone and antibiotics. The antibiotics most used were cefepime, azithromycin, ceftriaxone and piperacillin/tazobactam. Convalescent plasma was given to three out of the six patients. Four of the six (66.67%) subjects required invasive mechanical ventilation despite a robust and multifold treatment regimen.
5. Discussion
In our study, while the invasive mechanical ventilation was averted in 2 out of 6 patients, it is hard to draw a quantifiable conclusion regarding the efficacy of combination therapy due to the small sample size of our study population. It is interesting to note that multiple medications alone may not be the answer. Rather, a clear understanding of the underlying pathophysiology and appropriate time at which medications are administered, would prove more beneficial.
Data regarding the combination of remdesivir, IL-6 inhibitors, steroids, convalescent plasma and antibiotics is limited. Review of literature regarding combination therapy showed encouraging results though those reports were based on single individuals (20, 21). Anderson et al. (20) described successful management of an obstetric critically ill COVID-19 patient with a mixture of remdesivir, glucocorticoids and convalescent plasma. Coyle et al. (21) reported successful treatment and extubation of a COVID-19 patient with a disease course complicated by myocarditis and ARDS with a combination of corticosteroids, tocilizumab and experimental drug AT-001. Our study, albeit limited, questions whether there is any benefit in combining these treatment modalities.
Not only is the efficacy of combination therapy at question, but the safety profile of these combination regimens is a concern. There may be increased adverse effects with use of multiple medications during a similar time frame. Elevated aminotransferase levels are a documented adverse effect of remdesivir and tocilizumab (5, 15, 22) and some antibiotics as well. Of note, patients demonstrating aspartate transaminase (AST) and alanine transaminase (ALT) levels of five times the upper limit of normal or estimated glomerular filtration rate (GFR) of less than 50 mL/min formed the exclusion criteria in the remdesivir study by Goldman et al (8). No studies were found that compared the safety profile, compatibility or drug-to-drug interaction for remdesivir with tocilizumab (22). Currently, there are only a few clinical trials in the recruiting stage studying combination therapy (NCT04409262, NCT04410354, NCT04310228) that could advance our understanding in the future. NCT04409262 a randomized, double-blind study focuses on the safety and efficacy of the combination of remdesivir with tocilizumab compared to remdesivir with placebo in hospitalized patients with severe SARS-CoV-2 (23). NCT04410354 aims to study the combination of merimepodib, an antiviral and immunological agent that inhibits inosine monophosphate dehydrogenase with remdesivir in SARS-CoV-2 patients with a score of 3 or 4 on the National Institute of Allergy and Infectious Disease (NIAID) 8-point ordinal scale (24). NCT04310228 is another multi-center study that examines the efficacy and safety of tocilizumab combined with Favipiravir (25).
5.1. Limitations
Limitations of this case study include the limited sample size, non-uniformity of the use of medications, irregularity in timing of introduction of medications, the sequencing of the use of these drugs varied and finally underlying comorbid conditions may have played a confounding factor towards the outcomes.
5.2. Conclusion
Treatment modalities for COVID-19 appear to still be in its nascent stage. In our study, despite utilization of multiple drugs that have previously shown favorable results in other reports, invasive mechanical ventilation was thwarted only in 2 out of 6 study subjects. Due to the limited sample size, it is hard to draw any conclusive evidence that using multiple medications (remdesivir, IL-6 inhibitors, convalescent plasma, antibiotics and steroids) made any significant difference on stabilizing the patient and decreasing the need for invasive mechanical ventilation, morbidity and mortality. Combination therapy, however, remains intriguing and controversial. Many centers continue to use a variety of combinations of these medications without supporting data regarding the safety and efficacy profiles. Hopefully, ongoing studies will help further define the use of these medications and improve treatment outcomes. Judicious use of these medications in a methodical sequential use in patients that require three or more liters of supplemental oxygen is proposed. Use of any of these medications, either by itself or in combination, after mechanical ventilation has minimal if any benefits and should be used as a last resort.