1. Background
Malaria is one of the most severe public health challenges globally, it is the leading cause of morbidities and mortalities in many developing countries with young children (who have not developed partial acquired immunity to malaria), and pregnant women (whose immunities are decreased by pregnancy) are the most vulnerable groups (1). Malaria is a parasitic disease caused by Plasmodium species (P. falciparum, P. vivax, P. ovale, P. malariae and P. knowlesi) and transmitted mainly via the bites of infected Anopheles female mosquitoes but other minor forms of transmission are by sharing sharp objects, congenital malaria (mother to fetus), transfusion of infected blood and infected organ transplantation (2). According to the World Malaria Report, 228 million malaria cases occurred globally in 2018, and Nigeria accounted for 57 million (25%) cases (3). Also, there were 405,000 estimated malaria mortalities globally, with 97,000 (24%) occurring in Nigeria (3). The World Health Organization (WHO) recommends that a prompt, accurate laboratory diagnosis and treatment by skilled professionals is the most efficient strategy in curtailing asymptomatic malaria cases from progressing into severe cases and deaths (3). Laboratory diagnosis of malaria is mainly conducted by detecting Plasmodium species in clinical samples by microscopy, rapid diagnostic tests (RDTs) kits, and polymerase chain reaction (PCR) technique. Microscopic diagnosis of Plasmodium species in blood smears is the globally accepted “gold standard” for laboratory malaria diagnosis (4). However, low sensitivity or diagnostic accuracy, especially at low parasitemia, unavailable trained microscopists, absence of electricity and diagnostic equipment (such as microscopes, slides, etc.) are the major challenges to microscopy (5). The low diagnostic accuracy of microscopy, when conducted by individuals with little expertise, had led to malaria misdiagnosis globally (6). PCR technique can examine large clinical samples and detect mixed infections as well as has high specificity and sensitivity rates (7). Various PCR techniques have high levels of reliability in Plasmodium species diagnosis such as Nested PCR, Real-time PCR, and Reverse transcription PCR (8) but are not regularly used in most developing countries because they are expensive, complex to operate, and unavailable quality control. Several studies have been conducted in different countries, which highlight the importance of microscopy and PCR in detecting Plasmodium species; Nigeria (9), Tanzania (10), Indonesia (11), Saudi Arabia (12), and Mali (13). Misdiagnosis has led to wrong usage of antimalarial drugs and increased cases of malaria morbidities/mortalities globally.
2. Objectives
This study was conducted to evaluate the prevalence and misdiagnosis of Plasmodium using microscopy compared with PCR technique in two tertiary care hospitals in Rivers State, Nigeria.
3. Methods
3.1. Study Area and Population
A cross-sectional study was conducted in Rivers State University Teaching Hospital (RSUTH) and the University of Port Harcourt Teaching Hospital (UPTH). In fact, RSUTH is located at latitude 4°46'49"N and longitude 7°0'50"E in Port Harcourt Local Government Area (LGA), while UPTH is located at latitude 4°53'58"N and longitude 6°55'43"E in Obio-Akpor LGA (14). Port Harcourt and Obio-Akpor LGAs are in Rivers State, Nigeria. A sample size of 2,000 randomly selected participants (participants were of varying ages and both sexes) attending the Outpatients Clinics of the two selected healthcare facilities were recruited for this study (from January 2016 to December 2017). The inclusion criteria were informed consent to participating in the study, intermittent fever, abdominal pains, reduced/loss of appetite, and general body weakness, while the exclusion criteria were refusal to give informed consent, undergoing malaria treatment, and the presence of other febrile diseases.
3.2. Data Collection
Sample Preparation for Data Collection: Intravenous blood samples were collected by trained laboratory scientists and stored in Ethylene Diamine Tetra Acetate bottles to prevent coagulation.
Microscopic Diagnosis: Blood films (thick and thin) were prepared, stained (using Giemsa stains) from the collected blood samples, and visualized using X100 objective power lens of a microscope to detect the forms of Plasmodium present (15). Thick blood smears were used to quantify parasites (15).
DNA Extraction: The DNA from the blood sample of each study subject was first extracted using a Quick DNA Universal Kit (Zymo Research, USA). Blood sample (100 µl) from each study subject was put in separate microcentrifuge tubes, 100 µl of Bio-Fluid and Cell buffer and 10 µl of Proteinase K were added to each microcentrifuge tubes. The solution in the tubes were mixed and incubated for 10 minutes at 55°C to digest the blood samples. Genomic binding buffer (210 µl) was added to each of the digested blood samples and thoroughly mixed. The mixtures were transferred to Zymo-Spin Columns in collection tubes, centrifuged for 1 minute at 10,000 g, and the supernatant was discarded. About 20 µl of DNA Pre-Wash Buffer was added to the remaining mixture in new collection tubes, the columns were centrifuged at 10,000 g for 1 minute, and the supernatants in the collection tubes were discarded. About 350 µl of g-DNA Wash Buffer was added, centrifuged for a minute at 10,000 g, and the supernatants in the collection tubes were discarded. The remaining contents of the Zymo-Spin columns were transferred to clean centrifuged tubes; 50 µl of DNA Elution Buffer was added and centrifuged for 30 seconds at 10,000 g to elute the purified DNA in the supernatant. The ultra-pure DNA was preserved at < 0°C. Nanodrop 1,000 Spectrophotometer was used to quantify the extracted genomic DNA from each study subject.
Screening by Real-time PCR: Malaria diagnosis using the extracted DNA was conducted by Real-time PCR (ABI 9700 Applied Biosystems Thermal cycler). A universal primer that detected the four human species of Plasmodium was used; PL 1473 F (5'-TAA CGA ACG AGA TCT TAA-3') and PL 1679 R (5' - GTT CCT CTA AGA AGC TTT- 3') (16). The cocktail mix (25 µl) for each PCR reaction contained 12.5 µl Dream taq Master mix by Inqaba, South Africa (taq polymerase, DNTPs, MgCl), 0.4 µl of PL 1473 F, 0.4 µl of PL 1679 R, 4 µl of extracted DNA and 7.7 µl of PCR water. Reaction conditions for the amplification were initial denaturation (10 minutes at 95°C), denaturation (40 cycles for 10 seconds at 95°C), annealing (11 cycles for 5 seconds at 60°C with a touchdown at 0.5°C), extension (20 seconds at 75°C), final extension (2 minutes at 95°C), and cooling (30 seconds at 68°C).
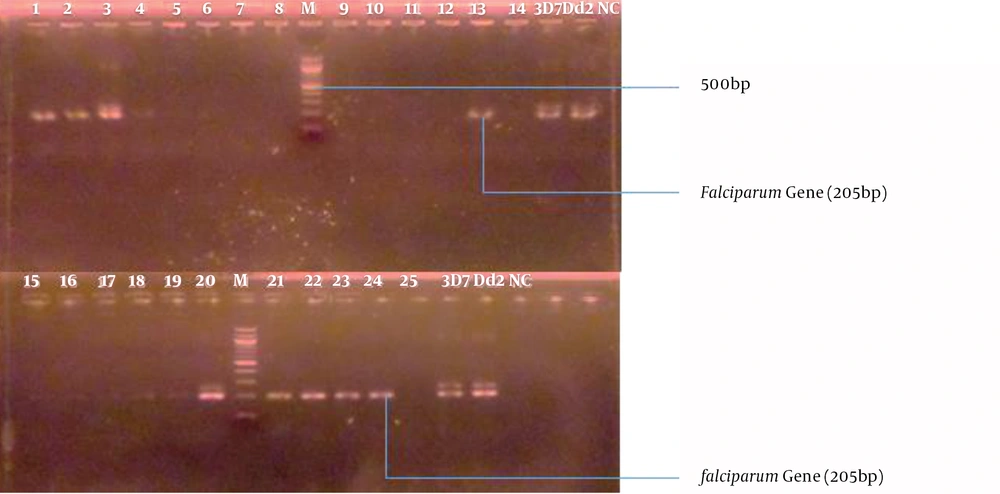
PCR Product Analysis by Agarose Gel Electrophoresis: The amplification products were viewed under UV transilluminator using agarose stained with 2% ethidium bromide at 120 V for 15 minutes (Figure 1).
3.3. Data Analysis
The data obtained for microscopy were evaluated using the PCR technique as the gold standard to determine variation, misdiagnosis, sensitivity, specificity, and diagnostic accuracy. Data were analyzed using the Chi-square test, and a P value of less than 0.05 was considered statistically significant.
4. Results
The only species of malaria parasite identified in this study by microscopy and with confirmation from polymerase chain reaction technique was P. falciparum. An overall prevalence of 38.0% and 34.0% were recorded for microscopy and PCR, respectively (P > 0.05) (Table 1).
| Healthcare Facility | Number Examined | Number Infected, (%) | |
|---|---|---|---|
| Microscopy | PCR | ||
| RSUTH | 1000 | 322 (32.0) | 283 (28.0) |
| UPTH | 1000 | 431 (43.0) | 397 (40.0) |
| TOTAL | 2000 | 753 (38.0) | 680 (34.0) |
Overall Prevalence of Malaria
An overall prevalence variation of 73 (4.0%) was recorded for microscopy compared with PCR and an overall misdiagnosis (overdiagnosis) of 5.5% was recorded in this study (P > 0.05) (Table 2).Overall malaria misdiagnosis for age groups 0-10, 11-20, 21-30, 31-40, and > 40 was 6.3%, 4.7%, 3.1%, 6.9% and 6.5%, respectively (P > 0.05) (Table 3). Overall malaria misdiagnosis for males and females was 6.5% and 4.5%, respectively (P > 0.05) (Table 3).
| Healthcare Facility (N = 2) | Number Examined | Number Infected Variation (%) |
|---|---|---|
| RSUTH | 1000 | 39 (4.0) |
| UPTH | 1000 | 34 (3.0) |
| TOTAL | 2000 | 73 (4.0) |
| RSUTH | ||
| Overdiagnosis, % | 5.4 | |
| Underdiagnosis, % | 0.0 | |
| UPTH | ||
| Overdiagnosis, % | 5.6 | |
| Underdiagnosis, % | 0.0 | |
| TOTAL | ||
| Overdiagnosis, % | 5.5 | |
| Underdiagnosis, % | 0.0 |
Diagnostic Prevalence Variation and Misdiagnosis of Microscopy
| Variable | Misdiagnosis | |
|---|---|---|
| Overdiagnosis, % | Underdiagnosis, % | |
| Age Groups (N = 5) | ||
| 0 – 10 | 6.3 | 0.0 |
| 11 – 20 | 4.7 | 0.0 |
| 21 – 30 | 3.1 | 0.0 |
| 31 – 40 | 6.9 | 0.0 |
| > 40 | 6.5 | 0.0 |
| TOTAL | 5.5 | 0.0 |
| Sex (N = 2) | ||
| Male | 6.5 | 0.0 |
| Female | 4.5 | 0.0 |
| TOTAL | 5.5 | 0.0 |
Overall Microscopy Diagnostic Variation According to Age Groups and Sex
In the overall study participants, P. falciparum was diagnosed in 73 (9.7%) by microscopy alone (not detected by PCR) and 680 (90.3%) were diagnosed as positive samples by microscopy and confirmed by PCR (Table 4). The overall diagnostic efficiency values in the study for sensitivity, specificity and diagnostic accuracy for microscopy when compared to PCR were 95.8%, 94.3%, and (94.9%), respectively (Table 5).
| Positive Parasite Diagnosis | Number Infected, (%) | Grand Total, (%) | |
|---|---|---|---|
| RSUTH | UPTH | ||
| Microscopy alone (not confirmed by PCR) | 39 (12.1) | 34 (7.9) | 73 (9.7) |
| Microscopy and confirmed by PCR | 283 (87.9) | 397 (92.1) | 680 (90.3) |
| TOTAL | 322 | 431 | 753 |
Positive Parasite Diagnosis by Microscopy
| Diagnostic Parameters | RSUTH (n = 1000)a | UPTH (n = 1000) | TOTAL (n = 2000) |
|---|---|---|---|
| Sensitivity | 95.6 | 95.9 | 95.8 |
| Specificity | 94.5 | 94.2 | 94.3 |
| Diagnostic accuracy | 94.8 | 94.9 | 94.9 |
Diagnostic Efficiency of Microscopy
5. Discussion
Plasmodium falciparum was the only species of malaria parasite recorded in this study. This finding is in agreement with reports from other studies that observed only P. falciparum (17-19). Malaria is endemic in most regions of sub-Saharan Africa and P. falciparum is responsible for 99.7% of estimated malaria cases in WHO African regions (where this study was conducted), 50.0% of cases in the WHO South-East Asia regions, 71.0% of cases in the East Mediterranean and 65.0% in the Western Pacific. The overall malaria prevalence of 38.0% in this study is comparable to 36.0% reported in Abia and 36.6% in Plateau (20), 40.8% in Rivers (21), 40.8% in Sokoto (22), 34.5% in Ogun (23), 38.7% in Kano (24), 31.6% in India (25), 35.7% in Kaduna (26) and 39.5% in Benue (27) but lower than 46.6% in Zamfara (28), 60.6% in Kano (29), 71.4% in Cross Rivers (30), 72.5% in Rivers (31), 78.0% in Southern Tanzania (32), 66.8% in Ogun (33), and 67.5% in Rivers (34). The study prevalence (38.0%) is higher than 14.7% reported in Lagos (35), 4.1% in Ethiopia (36), 20.7% in Lagos (37), 15.9% in Northwest Angola (38) and 11.4% in India (39). The low prevalence of malaria in this study, compared with higher prevalence values of previous malaria studies in Rivers State, might be attributed to increased public awareness and usage of insecticide treated nets as well as increased laboratory tests and prompt treatment of confirmed malaria-infected individuals. However, the malaria prevalence recorded in this study is of public health significance and it could be attributed to several factors in the study area such as environmental conditions (the temperature, humidity and altitude support the thriving of Anopheles species), blocked drainage systems (flooding and accumulation of stagnant water, especially after heavy rainfall, increases the competence levels and breeding of Anopheles mosquitoes) and over-crowded settlements (increases the chances of contracting malaria due to frequent physical contact between humans and mosquitoes). The overall diagnostic variation of microcopy was low when compared with the malaria diagnostic values of PCR. The diagnostic variations by microscopy in the study could be attributed to varying expertise among microscopists and errors in preparing or reading stained slides. The overall misdiagnosis of 5.5% in this study was relatively low; it was not statistically significant among age groups and sexes. The low value of misdiagnosis could be attributed to improved diagnosis and quality equipment in the tertiary hospitals as well as the successful efforts of the Rivers State Ministry of Health and Management of the studied healthcare facilities in improving the efficiency of malaria diagnosis by microscopy as one of the several tools in effective malaria control. Microscopists should be trained regularly to achieve high proficiency but PCR technique can further strengthen microscopy when Plasmodium identification is difficult. The overall positive sample diagnosis by microscopy and confirmed by PCR shows that microscopy remains the gold standard for malaria diagnosis when properly conducted but PCR diagnosis is also vital in sub-microscopic parasite densities.
5.1. Conclusion
The study provided data on Plasmodium species prevalence and misdiagnosis, which is necessary for effective malaria control strategies in endemic countries. The study also validated the use of microscopy by well-trained experts as a reliable Plasmodium diagnostic tool.