1. Introduction
Staphylococcus intermedius group (SIG) (1), which contains the bacteria S. intermedius, S. pseudintermedius, and S. delphini (2), is a coagulase-positive catalase-positive subset of the genre Staphylococcus, that exhibits beta-hemolysis on blood agar plates (3) and is commonly isolated in dogs (4), pigeons, visons, cats, horses, foxes, racoons, and goats (5).
Although differentiation between the three pathogens is possible with equivocal microbiological algorithms that use tests such as arginine-dehydrolase and mannitol fermentation, it is mainly carried out with techniques such as matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) (1, 6).
At a population level, SIG presents as a rare and transient member of skin and mucosal flora in humans. However, it can be frequently identified among dog owners and veterinary practitioners (5). Moreover, it has shown a pathogenic role in skin, and soft tissue wound infections (2, 3) - particularly in dog bite wounds- and has recently shown potential for rare invasive infections. Nevertheless, the true incidence of SIG in human wounds is probably underestimated due to its misclassification for Staphylococcus aureus (SAu) (2, 3) and case reports of invasive infections -endocarditis, catheter-associated infections, pneumonia, mastoiditis, sinusitis, and brain abscesses- are scarce (5).
We aim to present a case report of an invasive presentation of this pathogen that sheds light upon its high barrier for suspicion, driven by the underrecognition of its clinical importance.
2. Case Presentation
A 52-year-old male rural worker in contact with little and big animals, with a diagnosis of type 2 diabetes mellitus without treatment adherence, was admitted to the hospital. He reported 5 days of fever and malaise and presented with a symmetric cutaneous rash, temporally related to β-lactam antibiotic ingestion. Later on, during hospitalization, he complained of new-onset generalized myalgias. He had a history of potential exposure to rodent excretes at his workplace, which alerted physicians of the possibility of leptospirosis.
At the Emergency Department, the patient was febrile at 38.5°C, and his blood pressure and heart rate were 140/80 mmHg and 110 bpm, respectively. He initially required low supplemental oxygen with an inspired fraction of oxygen of 28%, reaching a pulse oximetry SatO2 of 96%. Physical examination revealed right-lower lobe pulmonary crackles at inspiration, without further relevant findings.
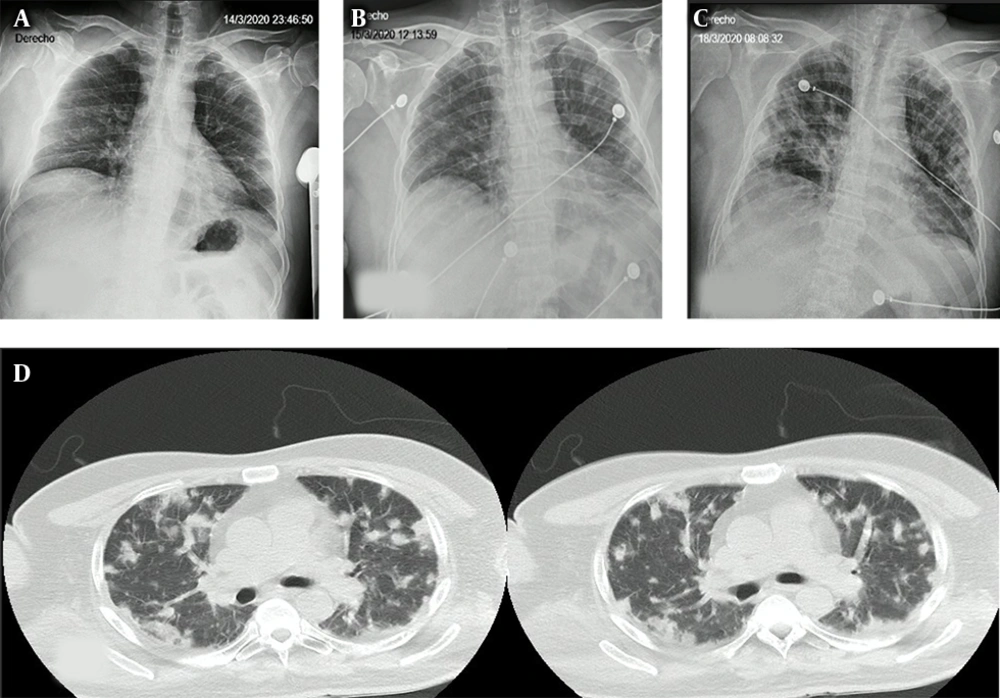
Blood samples were taken for cultures and routine blood investigations, apart from HIV and leptospirosis serologies (Table 1). In addition, a chest x-ray was performed that indicated atypical community-onset pneumonia (Figure 1A), and ceftriaxone was started at a dose of 1g every 12 hours.
| Variables | Admission to General Ward | Admission to Intensive Care Unit | Final stage of Hospitalization in Intensive Care Unit |
|---|---|---|---|
| Hemoglobin (g/dL) | 16.8 | 14.0 | 13.8 |
| White blood cells (cells/mm3) | 16.380 | 18.000 | 18.300 |
| Neutrophils (%) | 94.9 | 90.1 | 92.3 |
| Platelet count (plat/L) | 201.000 | 81.000 | 42.000 |
| Urea (mg/dL) | 49 | 66 | 75 |
| Creatinine (mg/dL) | 0.57 | 0.46 | 2.1 |
| Sodium (mEq/L) | 132 | 133 | 128 |
| Potassium (mEq/L) | 4.05 | 4.71 | 5.1 |
| Glycemia (mg/dL) | 316 | 219 | 310 |
| AST (U/L) | 60 | 57 | 81 |
| ALT (U/L) | 37 | 38 | 56 |
| ALP (IU/L) | 136 | 148 | 160 |
| Total bilirubin/direct bilirubin (mg/dL) | 0.47 | 0.48 | 2.5 |
| CK (U/L) | 992 | - | - |
| Albumin (g/dL) | 2.04 | - | - |
| Lactate (mg/dL) | - | 30.8 | - |
| pH | - | 7.36 | 7.26 |
| pCO2 (mmHg) | - | 35.5 | 53.9 |
| pO2 (mmHg) | - | 98 | 62.7 |
| HCO3- (mmHg) | - | 19.8 | 23.9 |
| Supplemental oxygen | FiO2 28% | FiO2 50% | Mechanical ventilation in prone position |
| Serologies | Hantavirus: negative; Leptospirosis: negative; HIV: negative; Syphilis: negative; Hepatitis B: negative | ||
| Ecocardiography | Slight increase in left atrium diameter; No vegetations; Ejection fraction 65%; Grade I mitral insufficiency | ||
Two days after arrival, he developed respiratory failure requiring admission to the intensive care unit (ICU) for frequent monitoring but without need for invasive mechanical ventilation (IVM) at first. At the time, he presented with bilateral pulmonary crackles, correlated to findings on a new chest x-ray (Figure 1C) and a posterior chest computed tomography (Figure 1D), which showed nodules in both lungs.
The Microbiology Laboratory informed bacterial growth in both blood culture bottles within 10 hours of incubation in a BacT/ALERT© (bioMérieux, US) automated microbial identification system. After the positive result, a sample was transferred to a solid growth medium, and catalase-positive Gram-positive Staphylococci colonies were identified.
Thus, the treating physicians added vancomycin at a dose of 1 g every 12 hours to empiric antibiotic treatment, suspecting SAu as the etiologic bacteria. With this presumptive diagnosis, a transthoracic echocardiogram was performed, with no evidence of vegetations. Afterward, the Microbiology Laboratory identified the etiologic bacteria as S. pseudintermedius through the VITEK® 2 Compact (bioMérieux, US) automated identification and antibiotic susceptibility testing system.
This result was manually confirmed with a positive pyrrolidonyl arylamidase enzyme (PYR) test as well as a positive O-nitrophenyl-β-D-galactopyranoside (ONPG) test and a negative Voges-Proskauer test. Methicillin resistance was detected with an oxacillin disk diffusion test. Due to an altered mental status and hypoxemia-driven high ventricular rate auricular fibrillation, he required orotracheal intubation for IMV during the ICU stay. In the process, mini-bronchoalveolar lavage fluid was obtained for culture, which later allowed for the diagnosis of methicillin-resistant S. pseudintermedius pneumonia. Despite all provided interventions, the patient died due to multiple organ failure.
3. Discussion
This case report displays one of the few descriptions of invasive infection by S. pseudintermedius. This microorganism colonizes the nares and anal mucosa of healthy cats and dogs, but it is also recognized as a veterinary pathogen (4, 6). Moreover, SIG is recognized as an infectious agent (7), particularly in special populations -namely people of old age as well as those with diabetes or immunodepression (3).
In a retrospective case series of 81 patients with SIG infections, only 7% reported contact with dogs, allegedly due to the under-registry of epidemiological data in clinical files. In 60% of investigated cultures in such series, the result was polymicrobial, failing to discriminate between the clinical characteristics of those patients with monomicrobial cultures versus those with polymicrobial cultures. Therefore, the clinical importance of SIG infections is unknown in polymicrobial settings (3).
Detailed clinical history with adequate epidemiological data recollection, with emphasis on occupational exposures, is key to our ability to elaborate papers of quality about these bacteria’s true incidence and dominant clinical presentations.
Adding complexity to the problem, SIG and SAu, apart from sharing clinical scenarios and risk factors, share morphological similarities that derive from frequent misclassifications of one for the other. This is the case, in particular, of settings without automatic microbiological identification systems (3); or without personnel with appropriate training and standardized algorithms for microbial identification (2). Therefore, true incidence cannot be ascertained until after these obstacles have been sorted out.
On top of that, cefoxitin disk diffusion tests, which are used to evaluate methicillin resistance in SAu, provide equivocal results with SIG when compared to results obtained with oxacillin tests. Hence, either ignorance about this fact or the misclassification of one organism for the other (3, 6), could derive from the premature de-escalation of antibiotics and poor clinical results. It is noteworthy that, despite the relatively low reported incidence of methicillin-resistance in SIG, the specimen isolated from our patient had a positive oxacillin test, as a surrogate for methicillin, without resistance to other investigated antibiotics.
In our center, we have only had four clinical isolates of SIG throughout last year, three of which were from blood cultures and one from a breast abscess. This is probably a reflection of the low rate of the culture of community-acquired skin and soft tissue infections in our hospital since most SIG isolates reported in the literature are from such samples (1, 3). In the case of our patient, the initial clinical diagnosis was that of community-acquired pneumonia. However, the epidemiological suspicion of leptospirosis, due to the contact of the patient with rodent excretes and the fact that this infection is endemic in our region, justified its investigation, although it was subsequently negative.
After that, complementary examinations, Gram stain results, and the torpid course of illness raised the suspicion of SAu bacteriemia; therefore, led us to adjust the empiric antibiotic therapy.
Although SIG pneumonia complicated with bacteriemia, which was the final diagnosis, never occurred to us in the list of differential diagnoses, the high standards of care of our Microbiology Laboratory and its fast results permitted the prompt arrival at such diagnosis. Nevertheless, it remains a question whether the first 48 hours without an appropriate antibiotic therapy was determinant for the patient’s demise, or if the course of the disease was marked solely by the pathogen’s aggressiveness.
3.1. Conclusions
Incorporating and producing knowledge about SIG infections, which can resemble SAu infections clinically and microbiologically, together with an exhaustive anamnesis, including detailed data about patients’ occupational and non-occupational exposures, will allow for fewer diagnostic mistakes involving this pathogen, therefore isolating SIG incidence from that of SAu.