1. Background
Group B beta-hemolytic streptococci (GBS) can cause serious infections in infants and pregnant women by colonizing in the lower gastrointestinal tract and urogenital tract. Infection with these bacteria is associated with several adverse pregnancy outcomes, including preterm delivery, the premature rupture of membranes, chorioamnionitis, neonatal pneumonia, and neonatal meningitis. The colonization of GBS is particularly prevalent in the rectovaginal area, and about 20 - 30% of the cultures prepared from pregnant women are GBS positive (1). Maternal infection is the most important cause of neonatal infections. The severity of neonatal infections depends largely on the degree of bacterial colonization in the mother (2, 3). Most serious clinical manifestations in newborns include pneumonia, bacteremia, and meningitis (4, 5). On the other hand, antibiotic use to prevent the infections caused by these bacteria has raised concerns about the emergence of drug-resistant bacterial strains. Resistance to erythromycin is thought to be caused by mutations in the gene encoding the L4 and L12 ribosomal proteins or a resistance factor that encodes the RNA23S subunit of a demethylating enzyme. Both changes result in poor binding of the antibiotic to the 50S subunit (6, 7). Due to the severity and life-threatening nature of GBS-associated infections in both the mother and fetus, it is necessary to evaluate the frequency of GBS colonization in pregnant women and to seek novel antimicrobials that are effective against resistant bacterial strains.
Given their favorable electrical, optical, and chemical properties, metal nanoparticles (NPs) have been extensively used in biotechnology and nanotechnology research. As one of the most important metal NPs with a high antimicrobial activity, gold nanoparticles (AuNPs) have been used in the industrial and medical scales in many countries. The infrared spectroscopy of free antibiotics and the antibiotics coated with AuNPs has shown that these NPs have a strong affinity for the structural rings of some antibiotics (8). In fact, AuNPs have a surface conjugate that is suitable for molecular probes and antibiotics. The conjugation of these nanoparticles with important biological molecules, such as oligosaccharides, DNA, and proteins, has been recently applied to label a variety of antibiotics such as gentamicin, streptomycin, neomycin, and carbapenems (9, 10).
2. Objectives
Due to the lack of data on the combined effects of AuNPs and erythromycin on infectious agents in Iran, this study aimed to determine the in vitro antimicrobial effects of erythromycin, either alone or in combination with AuNPs, on the GBS clinical strains isolated from pregnant women.
3. Methods
3.1. Bacteria Isolation
In this descriptive cross-sectional study, vaginal and rectovaginal swab samples were taken from 106 pregnant women aged 16 - 48 years (the mean age of 33 ± 9 years), referred to seven hospitals and maternity wards in Gorgan and its surrounding villages from January 2018 to May 2019. Written informed consent was obtained from all individuals before the study. The data related to gestational age, abortion history, number of previous pregnancies, employment status, ethnicity, place of residence, and economic status were collected using a questionnaire. The subjects had no history of systemic diseases, genitourinary tract infections, antibiotic consumption, and infectious diseases such as AIDS, hepatitis B, syphilis, toxoplasmosis, and cytomegalovirus infections.
Swabs were inoculated in Todd Hewitt broth (Sigma Aldrich, USA) containing 10 mg/L of colistin and 15 mg/L of nalidixic acid. After incubation at 37°C for 24 hours, the samples were cultured overnight in blood agar containing 5% sheep blood (Merck, Germany) at 37°C. Next, GBS strains were identified based on morphology, Gram staining, the catalase and CAMP tests, esculin and hippurate hydrolysis, and the SXT susceptibility test. For the genotypic examination of the isolates by PCR, the swab samples were first placed into phosphate-buffered saline (Merck, Germany) and kept in a freezer at -20°C until further testing. After the extraction of GBS genomic DNA using a commercial kit (Roche, Germany), PCR was carried out using the forward and reverse primers designed for amplifying the CFB gene: forward, 5' TTTCACCAGCTGTATTAGAAGTA 3'; reverse, 5' GTTCCCTGAAACATTATCTTTGAT 3'. Distilled water and pure GBS genome were used as the negative and positive controls, respectively.
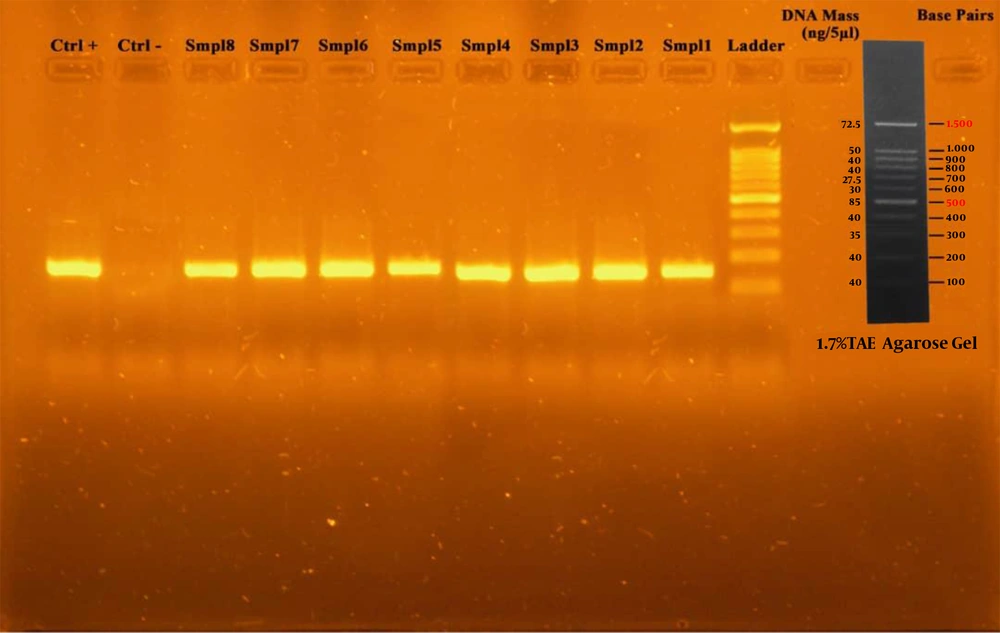
The PCR reaction was performed in a final volume of 25 μL, containing 1 μL of template DNA, 1 μL of each of primers, 0.05 μL of dNTP, 2.5 μL of PCR buffer, 0.75 μL of magnesium chloride, 0.2 μL of 5 U/μL Taq DNA polymerase, and 0.05 μL of deionized distilled water. Cycling conditions consisted of an initial denaturation at 94°C for 3 minutes, 1 minute of denaturation at 94°C with 40 cycles, and annealing at 55° C for 1 minute and extension at 72°C for 3 minutes (one cycle). PCR products were visualized by electrophoresis on 1.7% agarose gel. Finally, detection of a 153 bp band confirmed the identity of bacteria (Figure 1).
3.2. Determination of Drug Susceptibility
For this purpose, a GBS suspension in physiological saline with a turbidity of 0.5 McFarland standard was prepared from 24-hour cultures. The antibiotic resistance pattern of GBS isolates was determined by the disk diffusion (Kirby-Bauer) method using the following antibiotic disks: erythromycin (15 µg), penicillin (600 units/mL), ampicillin (10 µg), cefazolin (30 µg), clindamycin (2 µg), tetracycline (30 µg), and linezolid (10 µg). All antibiotic disks were purchased from PadTanTeb Co., Iran. After 18 - 24 hours of incubation at 37°C, results were analyzed according to the instructions described by the Clinical and Laboratory Standards Institute (CLSI M100-S25) (11).
3.3. Determination of Minimum Inhibitory Concentration of Erythromycin by Broth Microdilution Method
In order to prepare the drug stock, erythromycin powder (Sigma Aldrich, USA) was dissolved in 95% ethanol to obtain a final concentration of 8 μg/mL. The antibiotic was inoculated into the first and second wells of a 96-well ELISA microplate containing Mueller-Hinton broth (Merck, Germany). Simultaneously, with the inoculation of the bacterial suspension with a concentration equivalent to 0.5 McFarland standard, serial dilutions of the drug were prepared in the range of 8 - 0.01 μg/mL. Finally, the microplate was incubated at 37°C for 20 - 24 hours. Wells without the bacterial suspension were considered blank (the negative control), and the wells containing the bacterial suspension were considered the positive control. The lowest concentration of the antibiotic that inhibited the growth of 90% of bacteria was considered MIC90. The inhibitory effect was assessed by reading the absorbance at 630 nm using an ELISA Reader (BioTec, Germany). According to CLSI instructions, strains with a MIC of ≤ 0.25 μg/mL, equal to 0.5 μg/mL, and ≥ 1 μg/mL were considered sensitive, semi-sensitive, and resistant to erythromycin, respectively (10). The standard Streptococcus pneumoniae 49619ATCC strain was used as a control.
3.4. MIC of the AuNPs-erythromycin Combination by Broth Microdilution Method
Liquid AuNPs with the amine groups sized 30 nm were purchased from Nanopishgaman Co., Iran. To prepare the stock solution, 20 mL of AuNPs (3 mg/mL) was mixed with 10 mL of erythromycin (8 μg/mL) for four hours in a shaker incubator. After centrifugation at 3800 rpm, the precipitate was dissolved in a mixture of distilled water and ethanol. To determine the MIC of AuNPs, either alone or in combination with erythromycin, 100 μL of the stock AuNP-erythromycin solution was inoculated into the wells of a 96-well plate containing Mueller-Hinton broth (Merck, Germany). Simultaneously, with the inoculation of the bacterial suspension (3 × 108 CFU/mL), serial dilutions were prepared from AuNP-erythromycin in the range of 0.01 - 8 μg/mL. Finally, the microplate was incubated at 37°C for 20 - 24 hours.
3.5. Determining the Antibacterial Effect of AuNP-erythromycin
A bacterial suspension with a concentration equal to 0.5 McFarland standard was prepared from all erythromycin-resistant GBS isolates that were detected by the agar well-diffusion method. The isolates were cultured on Mueller-Hinton broth with 5% sheep blood. Then three wells with a diameter of 7 mm were created on the medium using a sterile pasteurizer pipette. Next, 100 μL of each of AuNPs (control), erythromycin (control), and the AuNP-erythromycin combination was poured into the wells. The plate was incubated at 37°C for 24 hours. The presence of a growth inhibition zone with a diameter of greater than 12 mm and less than or equal to 10 mm indicated sensitivity and resistance, respectively.
3.6. Statistical Analysis
Data were expressed as mean ± standard deviation. All data were analyzed by SPSS software (version 23) using the independent t-test and Fisher's exact test. Inter-group comparisons were performed using one-way analysis of variance. A p-value of less than 0.05 was considered statistically significant.
4. Results
4.1. Demographic Information and Prevalence of GBS Isolates in Pregnant Women
Out of 106 samples, 23 (21.7%) were identified as GBS. The prevalence of the isolates was the highest in the subjects aged < 40 years (73.9%) (P = 0.0251), those with a history of abortion (60.9%) (P = 0.038), employed women (65.2%) (P = 0.069), Turkmen ethnicity (65.2%) (P = 0.098), women with a history of three or more pregnancies (52.2%) (P = 0.06), and those living in rural areas (60.9%) (P = 0.038).
4.2. Results of the Disk Diffusion Test
Based on the results, 15 (65.2%) GBS isolates were resistant to erythromycin, while the highest sensitivity was related to linezolid (87%) (Table 1). According to the independent t-test, there was a significant association between the type of antibiotic and its effect size (P = 0.041). Our analysis indicated a significant relationship between sensitivity to erythromycin and some variables, including age, employment status, history of abortion, and place of residence.
| Drug Class | Antibiotic | Relative Frequency | ||
|---|---|---|---|---|
| Resistant | Intermediate | Susceptible | ||
| Oxazolidinones | Linezolid | 8.7 | 4.3 | 87 |
| Lincosamides | Clindamycin | 34.8 | 26.1 | 39.1 |
| Penem | Penicillin | 60.9 | 7.21 | 17.4 |
| Cephams | Cefazolin | 21.7 | 8.7 | 69.6 |
| Macrolides | Erythromycin | 65.2 | 0 | 34.8 |
| Cyclines | Tetracycline | 39.1 | 17.4 | 43.5 |
4.3. Results of the Broth Microdilution Test
There was a significant difference in the MIC50 (4 μg/mL) and MIC90 (16 μg/mL) of erythromycin against GBS isolates (P = 0.02). No growth was observed at the concentration of 2 μg/mL of erythromycin. The combination of AuNPs with erythromycin significantly increased the antimicrobial activity of the antibiotic against erythromycin-resistant GBS isolates (Table 2). As shown in Table 3, the mean diameter of growth inhibition zone for AuNP-erythromycin was greater than that of AuNPs and erythromycin alone (P = 0.037).
| Variables | Erythromycin | Erythromycin+ AuNPs | P-Value |
|---|---|---|---|
| Age | 0.036 a | ||
| < 40 | 2 (11.8) | 15 (88.2) | |
| > 40 | 0 | 6 (100) | |
| Rank of pregnancy | 0.027 a | ||
| First | 0 | 3 (100) | |
| Second | 1 (12.5) | 7 (87.5) | |
| ≥ Third | 1 (8.3) | 11 (91.7) | |
| Abortion | 0.059 | ||
| Yes | 1 (7.1) | 13 (92.9) | |
| No | 0 | 9 (100) | |
| Occupation | 0.02 a | ||
| Yes | 1 (6.7) | 14 (93.3) | |
| No | 0 | 8 (100) | |
| Place of residence | 0.063 | ||
| City | 1 (11.1) | 8 (88.9) | |
| Village | 0 | 14 (100) | |
| Ethnicity | 0.04 a | ||
| Fars | 0 | 8 (100) | |
| Turkmen | 1 (6.7) | 14 (93.3) |
a P < 0.05 (significant).
5. Discussion
Group B streptococci are present in the rectal and vaginal microflora of more than 35% of women. These bacteria are important causes of infections in pregnant women and infants (12). In our study, the frequency of GBS isolates in pregnant women in Gorgan was 21.7%. In previous studies in Iran, the frequency of GBS among women ranged between 5.3 and 81% (13-15). The frequency of GBS was reported to be 54.9% in Mexico (2), 17.2 - 20% in Poland (7), 16% in Israel (16), and 12.9% in Thailand (17). The difference in the frequency rates can be related to variations in sampling techniques, methodology, the number of sexual partners, and the rate of using antibiotics. Despite the administration of antibiotics according to CDC guidelines to prevent maternal GBS transmission, the rate of GBS-associated infections is still increasing. In addition, the rate of erythromycin resistance has significantly increased to as high as 30% in the last three decades (18). In our study, the prevalence of erythromycin-resistant GBS isolates was 65.2%, which was notably higher than the rates reported in the United States (30%), France (21.4%), Turkey (20%), and Portugal (10.7%) (19-21).
Our analysis indicated a significant relationship between sensitivity to erythromycin and some variables, including age, employment status, history of abortion, and place of residence. Inconsistent with this finding, previous studies in Iran (14) and Tunisia (22) reported no significant relationships between GBS colonization and maternal age, education level, nationality, and parity.
Due to the increasing rate of drug-resistance, prevention of GBS-related infections and timely treatment are essential to ensure the safety of both the mother and infant. The advantages of using NPs instead of antibiotics include the lack of bacterial resistance and having no side effects for humans (23, 24). In recent decades, AuNPs have been used as potent antibacterial agents to eliminate drug-resistant bacteria. The efficiency of these NPs depends on their sizes and doses so that the antibacterial activity of NPs is the highest when they have a size in the range of 6 - 40 nm. It has been demonstrated that the conjugation of AuNPs with antimicrobial compounds can deliver a more potent antimicrobial activity. For instance, the AuNPs conjugated with vancomycin had a 50-fold higher antibacterial activity against vancomycin-resistant enterococci compared to vancomycin alone (25). Furthermore, Williams et al. showed that AuNPs alone did not have any antibacterial effect, but the AuNPs conjugated with vancomycin had inhibitory effects against bacteria (26). Other studies also reported the synergistic effect of AuNPs in conjugation with antibiotics such as carbapenems (10) and ciprofloxacin (27) against a wide range of microorganisms. In the present study, we observed a relatively potent antibacterial effect for AuNPs against GBS isolates. In fact, the AuNPs combined with erythromycin could eliminate most erythromycin-resistant streptococcal strains. This effect was 2.5-fold higher than the antimicrobial effect of erythromycin alone. The synergistic effect of AuNPs and erythromycin could be related to the ability of these NPs to modulate various vital cellular functions such as energy production, microbial cell movement, and protein synthesis (28).
5.1. Conclusion
Based on our results, the prevalence of GBS and erythromycin resistance was high among pregnant women in Gorgan, Iran. The combination of AuNPs with erythromycin showed a significantly enhanced antimicrobial activity against resistant GBS isolates compared with each of AuNPs and erythromycin alone.