1. Background
Cardiovascular diseases (CVDs), like many other chronic diseases, are closely related to lifestyle, mental health, and quality of life, which can lead to other diseases if not controlled (1). In 2016, according to the World Health Organization (WHO), CVDs accounted for 56.9% of 15.2 million deaths, i.e., almost one in three (2). Also, the prevalence of CVDs is higher in the Middle East than in other parts of the world (3). Epidemiological studies in Iran and the United Arab Emirates indicated that the prevalence of CVDs will reach 5.9% (4). Lifestyle alteration has led to the widespread prevalence of obesity and overweight, which is the most important risk factor for CVDs (5, 6). According to the literature, physical activity level (PAL) and exercise are simple and low-cost non-pharmacological interventions to prevent risk factors for CVD and improve the quality of life of CVD patients (7). Studies have shown that increased PAL reduces the inflammatory biomarkers and CVD risk factors (8, 9). On the other hand, in late December 2019, the first variant of the new coronavirus, COVID-19, was identified (10). According to the literature, age and underlying diseases, including hypertension, diabetes, CVD, etc., are closely related to the severity of COVID-19 and the occurrence of the acute respiratory syndrome (11, 12). The low PAL induced by COVID-19 quarantine increases the risk factors for CVDs (13). Until finding a definitive cure, strengthening the immune system is one way to prevent or minimize the adverse effects of COVID-19, which can be achieved by promoting PAL (14). Exercise has positive effects on the immune system and has been shown to decrease the negative effects of COVID-19 quarantine on the immune system. Regular exercise not only increases the ability to perform daily tasks and functional capacity but also strengthens the immune system of CVD patients (15, 16). Due to the novelty of the coronavirus, so far, limited scientific studies have evaluated the effect of exercise on COVID-19, particularly in CVD patients.
2. Objectives
Considering the importance and role of PAL in preventing or minimizing the risk of infectious diseases in CVD patients, this study aimed to investigate the association between the COVID-19 incidence and PAL in CVD patients during the COVID-19 outbreak.
3. Methods
3.1. Study Design
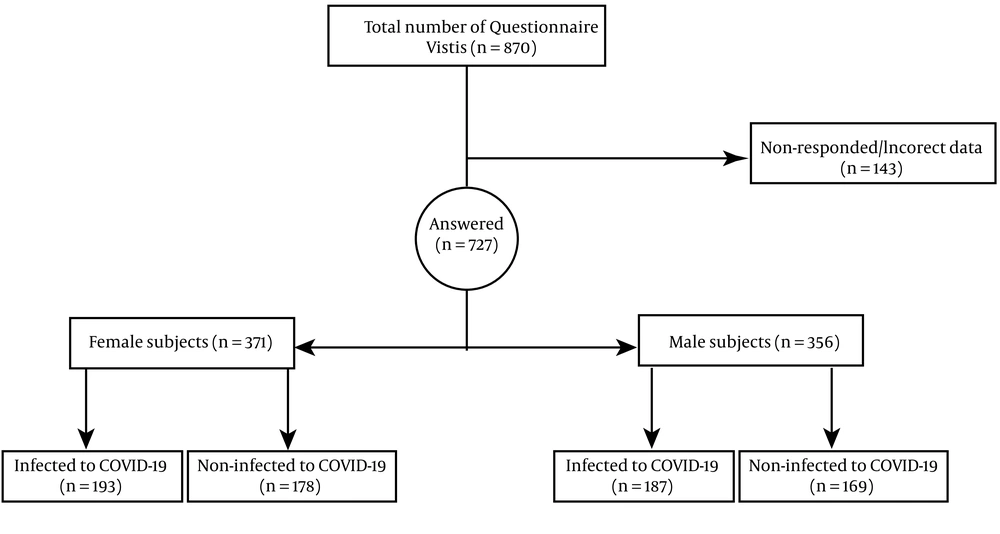
The statistical population of this cross-sectional-descriptive study consisted of all patients with CVD in Kermanshah. Using Cochran's sample size formula, with a 95% confidence level and 99% test power, the sample size was determined as 384 subjects. The questionnaire was sent to 870 available samples, of which 727 individuals filled the questionnaire (371 men and 356 women).
3.2. Population
Inclusion criteria included: Men or women aged ≥ 30 years and ≤ 70 years, diagnosis of hypertension (i.e., systolic and diastolic blood pressure over 140 and 90 mmHg) or using blood pressure-lowering medications, stable typical or atypical symptoms consisting of jaw pain, chest pain, shoulder pain, palpitations, chest tightness, chest burning, non-chest pain symptoms (e.g., dyspnea or worsening effort tolerance), atherosclerosis diagnosis, and angina diagnosis using computerized tomography (CT) scan or magnetic resonance angiography (MRA). Also, the exclusion criteria were: Partially filled questionnaires, smoking, having other cardiac problems such as arrhythmia and valvular disease. The objectives and importance of the research were explained to the participants before answering the questionnaires; observing ethical considerations was also assured (Figure 1).
3.3. International Physical Activity Questionnaire
International Physical Activity Questionnaire (IPAQ-SF) is designed to collect information on the time spent being physically active during the last week in four categories: Vigorous-intensity, moderate-intensity, walking, and sitting (17, 18). The activity patterns in each category should be multiplied by their estimated value in Metabolic Equivalent of Tasks (METs). Then, the scores of four categories were summed to estimate the overall physical activity in a week. The MET intensity values were 8 METs in vigorous, 4 METs in moderate, 3.3 METs in walking, and 1 METs in sitting (19). PAL scores > 3000 were considered as high, between 600 - 3000 as moderate, and < 600 as low (20). Kelishadi et al. confirmed the validity and reliability of the questionnaire in a sample of Iranians (21).
3.4. Statistical Analysis
Data analysis was administered using SPSS version 24. Statistical significance was considered when the P-value < 0.05. The Pearson correlation coefficient and independent t-test were used to evaluate the association between variables and to compare the mean value of variables between men and women, respectively.
4. Results
Table 1 shows the demographic characteristics, PAL, and the incidence of COVID-19. The results show that women have lower PAL than men; women also had a higher incidence of COVID-19 (Table 1).
| Variables | Men (n = 356) | Women (n = 371) |
|---|---|---|
| Age (y) | 43.70 ± 8.34 | 46.18 ± 6.32 |
| Height (cm) | 171.03 ± 11.14 | 160.23 ± 7.17 |
| Weight (kg) | 83.24 ± 8.16 | 76.44 ± 5.23 |
| BMI (kg/m2) | 28.46 ± 6.24 | 29.85 ± 4.46 |
| PAL (MET) | 1023.11 ± 23.12 | 982.32 ± 29.15 |
| Incidence of COVID-19 (%) | 52.02 | 52.52 |
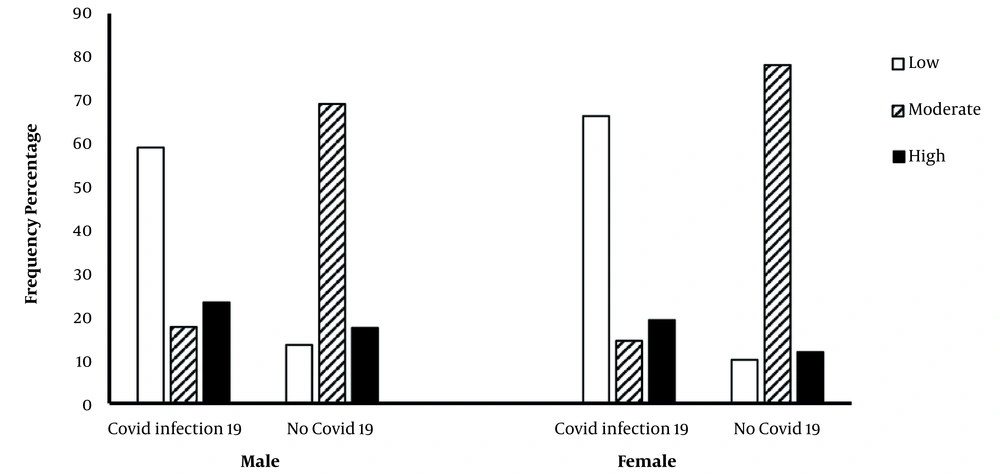
The results indicate that most CVD patients with COVID-19 had low PAL, while a lower percentage of them had moderate PAL. Interestingly, most non-infected CVD men and women had moderate PAL (Figure 2).
Also, there was a significant positive association between low and high PALs and the incidence of COVID-19 in men and women with CVDs. There was a significant inverse association between moderate PAL and the incidence of COVID-19 (Table 2).
5. Discussion
The overall mean physical activity level (PAL) of the subjects was moderate. Although not statistically significant, the PAL was higher in men than women. According to results of previous studies, various exercise activities (e.g., resistance, aerobic, and combined) increase the cardiovascular fitness of men and women at all ages (from childhood to elderly age), which indicates a direct association between physical activity and risk factors for CVDs (22, 23). Physical activity, on the one hand, strengthens the autonomic system (sympathetic/parasympathetic imbalance) function and, on the other hand, reduces the disturbance of ventricular repolarization, leading to attenuated ventricular function and sudden death (22, 24). Also, physical activity reduces the risk of cardiac arrhythmias by improving endothelial function, reducing platelet adhesion, and modifying the autonomic system (24). The findings showed that most CVD patients (both men and women) with COVID-19 had low PAL. However, a lower percentage of CVD patients with moderate PAL were infected with COVID-19.
In other words, the present study showed that most non-COVID-19 CVD patients had moderate PAL. According to previous studies, moderate PAL reduces the risk of infectious diseases, including viral and bacterial infections (25, 26). Moderate PAL also affects the immune system by invoking lymphoid from the tissue stores of myokines, glucocorticoids, and hormones (e.g., enkephalin) (27). This evidence demonstrates the ability of moderate PAL in improving the immune responses to infections and cancer and maintaining the immune system (28, 29). Interestingly, our results also show a significantly positive relationship between the incidence of COVID-19 with low and high (vigorous) PAL in both men and women with CVD. According to previous studies, high PAL leads to inadequate recovery and may cause temporal immunosuppression (attenuating the immune system) state (30). Vigorous exercise can alter or suppress some immune parameters, such as circulating leukocytes, plasma cytokine concentrations, salivary immunoglobulin A secretion, macrophage, and neutrophil xenophobia. Therefore, high PAL is associated with an increased risk of infection (30, 31). Previous studies indicate decreased immune system parameters (e.g., serum immunoglobulin) and antibody production following vigorous exercise (32). There are different mechanisms associated with the effect of PAL on antibodies and serum immunoglobulins. The difference in the ratio of antibody secretion and degradation might be due to the change in the concentration of serum antibodies that could be due to vascular abnormalities. On the other hand, exercise increases the lymph flow, leading to altered protein entrance to the bloodstream (33). Also, the half-life of serum immunoglobulins is affected by the exercise variables (including severity and duration), resulting in a difference in the number of antibodies and serum immunoglobulins (34).
5.1. Conclusions
In general, moderate PAL is associated with a lower incidence of COVID-19, probably by strengthening the immune system. Thus, it is recommended that individuals with underlying diseases (e.g., CVD) have moderate PAL during COVID-19 quarantine while observing COVID-19 restriction protocols.