Dear Editor,
Global vaccination (regardless of the type of vaccine) aligned with adhering to the health protocols (wear a mask, use hand sanitizer, social distancing, etc.) is the most efficient strategy to seize this pandemic (1). The COVID-19 pandemic has caused irreparable economic and social damage to health care systems. Asymptomatic patients and patients in the early stages of the disease may not know that they are infected and take action to get the vaccine. In consequence, what would be happened to them? Is it hazardous to get a vaccine when we are unsure about the infection?
The incubation period of COVID-19 is varied according to the variants. Delta variant has a shorter period (it takes four days from exposure to positive PCR result), but it was six days (2). Accordingly, some patients are asymptomatic and may represent happy hypoxemia, which is high risk (3). Since vaccination has started in countries, it is possible to vaccinate people who are in the incubation period of COVID-19 or asymptomatic, and it is hypothesized that vaccination can trigger immune systems, which may lead to critical status. COVID-19 vaccine companies notify you that the vaccine has side effects, which some of which can be severe and even like coronavirus infection. There is an overlap between the side effects of vaccines and possible virus infection after receiving the vaccine. Yet, we do not have a protocol to differentiate them from each other.
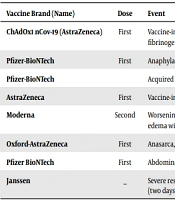
There are some reports of eventful consequences and death after COVID-19 vaccination worldwide (Table 1).
| Vaccine Brand (Name) | Dose | Event | Country |
|---|---|---|---|
| ChAdOx1 nCov-19 (AstraZeneca) | First | Vaccine-induced isolated carotid arterial thrombosis with a normal level of platelet count and fibrinogen | Germany (4) |
| Pfizer-BioNTech | First | Anaphylactic reaction to polyethilenglicole (PEG) | Italy (5) |
| Pfizer-BioNTech | Acquired hemophilia A | Saudi Arabia (6) | |
| AstraZeneca | First | Vaccine-induced immune thrombotic thrombocytopenia | Saudi Arabia (7) |
| Moderna | Second | Worsening shortness of breath, fever, and chills. Chest X-ray showed cardiomegaly and pulmonary edema without any focal consolidation, which is associated with acute myocardial injury. | USA (8) |
| Oxford-AstraZeneca | First | Anasarca, acute kidney injury (AKI), and severe hypertension (13 days after vaccination). | Canada (9) |
| Pfizer BioNTech | First | Abdominal MRI presented mild pancreatic injury (a day after vaccination). | Poland (10) |
| Janssen | _ | Severe respiratory distress and acute hypoxic hypercapnic respiratory failure and septic shock (two days after vaccination). | USA (11) |
Events After Vaccination
It is believed that vaccines may trigger the immune system and cause severe adverse effects. However, all mentioned reactions and adverse effects cannot be linked to vaccination. Therefore, any association or casualty needs to be confirmed by more studies.
As the side effects and risks of vaccination were noted, some data about the effectiveness of vaccines should be provided. In Doha, Qatar, 1.7 % of people (6689 out of 385,853) who received the first dose of the mRNA vaccine BNT162b2 (Pfizer–BioNTech) and 0.6 % (1616 out of 265,410) of people who received the second dose were infected, and five persons after the first dose and two after the second dose died by COVID-19, respectively (12). A test-negative case-control study in England reported that the effectiveness of used vaccines (BNT162b2 and ChAdOx1 nCoV-19) after the first and the second dose in the alpha variant was 48.7 and 87.5 compared to 30.7 and 79.6 in delta variant (13). In Israel, during the period of 14 to 20 days after the first dose of the BNT162b2 mRNA vaccine, the estimated vaccine effectiveness for documented infection was 46%, for symptomatic COVID-19 illness was 57% for hospitalization was 74%, for severe illness was 62%,; and for death was 72%. During the period of 21 to 27 days after the first dose, the estimated effectiveness for these outcomes was 60%, 66%, 78%, 80%, and 84%, respectively. In the follow-up period starting seven days after the second dose, the vaccine effectiveness for documented infections, symptomatic illness, hospitalization, and severe disease was 92%, 94%, 87%, and 92%, respectively (14). Accordingly, COVID-19 vaccines are highly potent and effective in preventing hospitalization and death. Therefore, it is suggested that vaccination is the best way to reduce the consequences of COVID-19 currently.
Screening people before administrating the vaccine is crucial for the vaccination protocol. The Center for Disease Control and Prevention (CDC) (15) proposed a questionnaire called “Prevaccination Checklist for COVID-19 Vaccines” for people who want to receive the vaccine and they have to fill it to ensure whether they are eligible to vaccinate or not. The Ministry of Health, Labor, and Welfare (MHLW; Japan) (16) and Health Department of Queensland government (Australia) (17) render a checklist for prevaccination screening (in Iran, they ask about age, weight, height, background disease, and any history of COVID-19 infection in the past month).
We believe that global vaccination is a secure way to end the pandemic to the best of our knowledge. It is undeniable that coronavirus vaccines (just like other medications at the first step of use) have some side effects. However, if we consider the proportional risk to reward globally, it is advisable to vaccinate people worldwide to end the COVID-19 pandemic. To avoid vaccination of infected people, it is suggested that a more comprehensive screening tool should be utilized. Checking complete blood counts differentiated (to assess lymphopenia) and C-reactive protein (to assess active inflammation) some days before vaccination seems to be an appropriate approach. This is the primary hypothesis, and further studies are needed to confirm it.