1. Background
The emergence of antimicrobial resistance and its negative impact on patient survival and associated healthcare costs is a growing global public health problem. Antibiotics account for about 20 - 30% of total drug expenditures, which is one of the highest drug costs worldwide (1). For instance, antibiotic expenditures were estimated at $56.0 billion in the United States within 2010 - 2015 (2). Irrational antibiotic consumption not only increases the drug expenditures but also can result in the unnecessary drainage of already limited resources in developing countries. It has been estimated that antimicrobial resistance would result in an approximately 1% annual decrease in global gross world product (GDP), and there would be a 5 - 7% loss in GDP in developing countries by 2050 (3).
The use of a significantly high percentage of prescribed antibiotics has been reported to be inappropriate or unnecessary (4). There is an urgent need to promote rational antibiotic prescription to decrease antibiotic resistance, which in turn can lead to reduced length of hospitalization, mortality, and healthcare expenditures. Surveillance of antibiotic consumption in hospitals can identify areas for the improvement of antibiotic use that has the potential to decrease healthcare costs by reducing the incidence of antimicrobial resistance, antibiotic-associated adverse events, and expenditures due to inappropriate or unnecessary antibiotic consumption.
The anatomical therapeutic chemical/defined daily dose (ATC/DDD) system, suggested by the World Health Organization (WHO), is becoming a standard method used to assess drug consumption by institutions and facilitates benchmarking within and among hospitals (5). The DDD is a technical unit of measurement that has been described as “the assumed average maintenance dose per day for a drug used for its main indication in adults” (5). The DDD per 100 bed-days is the most frequently used metric for the quantification of antibiotic use in the hospital setting (6).
Antibiotic overuse and misuse and poor compliance with guidelines for antibiotic therapy (in some cases less than 30%) have resulted in a rise of antimicrobial resistance in Iran (7, 8). It has been reported that antibiotic consumption in Iran is three times the average antibiotic consumption in the Organization for Economic Co-operation and Development countries (9). However, the studies performed on antimicrobial utilization and prescription patterns in Iran are very limited, and there is insufficient information about antibiotic consumption patterns in Iran’s hospitals.
2. Objectives
The current study aimed to describe the patterns of antibiotic use in a tertiary care teaching hospital in Zahedan, southeast of Iran, using the ATC/DDD system suggested by the WHO.
3. Methods
This cross-sectional study was conducted in Ali-Ibn-E-Abitaleb (peace be upon him) teaching hospital, which is a 523-bed tertiary care and referral center in Zahedan, southeast of Iran. The clinical departments in this hospital comprise six intensive care units (ICUs), seven medical (four adults and three pediatrics) and four surgical wards, one obstetrics and gynecology ward, one radiotherapy, and one hemodialysis unit.
Only acute care inpatient wards in the hospital were included in this study, and it was decided to exclude the patients admitted to pediatrics wards and ICUs. Therefore, patients who were hospitalized in seven general surgical and nonsurgical wards, including four internal medicine (ie, internal medicine ward No. 1, internal medicine ward No. 2, internal medicine No. 3, and hematology), two surgical wards (ie, women’s surgery and men’s surgery), and one obstetrics and gynecology ward, were eligible for inclusion in the study on the day of discharge. The patients admitted to the internal medicine wards No. 2 and No. 3 were mostly but not merely medical oncology-endocrinology and pulmonology-gastroenterology-rheumatology patients, respectively.
For the period of March 2017 to March 2018, the data of the patients admitted to the above-mentioned wards were collected by reviewing the records of discharged patients and retrieving data from the hospital health information system (HIS). According to the WHO guidelines for drug utilization research, 75 patient records were randomly selected from each ward (ie, a total of 525 patients from seven wards) (5). Stratified random sampling was used for selecting patients. Patients 18 years of age and older, whose duration of hospitalization in medical and surgical wards was 5 and 3 days and longer, were included in this study, respectively.
The patient-specific data, such as demographics, underlying disease states, type of patients’ admission, name of antibiotic, dosage, antibiotic administration route, duration and indications for antibiotic use (e.g., prophylaxis, empirical, and targeted), antibiotic prescriber’s specialty, antibiotics costs, type and date of surgery if any was performed, and patient outcomes, were collected. The data on the number of active hospital beds and bed occupancy percentage for each ward for the study period were obtained monthly from the hospital statistics center.
The ATC/DDD system codes 2020 (ATC group J0) were used to determine antibiotics for systemic use (5). The antibiotic consumption data were converted to DDD and expressed as defined daily dose per 100 bed days (DBD) for the use of individual antibiotics, classes of antibiotics, and total antibiotic consumption using the following formula (5):
Descriptive statistics were used for data analysis. The prevalence of antibiotic prescription was calculated as the proportion of the patients receiving any antibiotic out of the total number of studied patients. The 10 most commonly used antibiotics (top 10) were identified based on the calculated annual DBD for each antibiotic. All continuous variables were tested for the normality of distribution using the Kolmogorov-Smirnov goodness of fit tests. None of the continuous variables was observed to have a normal distribution. Therefore, nonparametric tests were used for data analysis. The mean values of 12 months of DBD for overall antibiotic consumption were compared between different hospital wards using the Kruskal-Wallis test. A p-value less than 0.05 was considered significant for all analyses. Data analysis was performed using SPSS statistical software package (version 20; Chicago, IL, USA).
4. Results
In this study, the medical records of the patients admitted to seven wards in Ali-Ibn-E-Abitaleb (peace be upon him) teaching hospital were investigated for antibiotic consumption. The average number of active beds in these wards and the average percentage of bed occupancy were 22.4 and 94.1, respectively.
A total of 2808 patients were admitted to these wards during the study period, of whom 525 patients (41% males and 59% females) were randomly selected for this study. More than half of the study population were 50 years of age and older. Overall, 90% of the studied patients were discharged. Moreover, 7% of the patients were discharged against medical advice, and 3% of the patients died.
The total percentage of patients receiving antibacterial was 73.1% (Table 1). The highest proportion of the patients on antibacterial therapy was among cases admitted to the obstetrics and gynecology ward (96%), followed by surgical wards (82.7% and 81.3%). Furthermore, the lowest rate of 48% was observed in the internal medicine ward No. 2 (ie, medical oncology-endocrinology). Among 384 patients who were treated with antibiotics, 100 (26%), 130 (34%), and 154 (40%) patients received 1, 2, and 3 agents or more antimicrobials, respectively.
| Ward | No. (%) |
|---|---|
| Obstetrics and gynecology | 72 (96.0) |
| Men’s surgery | 62 (82.7) |
| Women’s surgery | 61 (81.3) |
| Internal medicine ward No. 3 | 52 (69.3) |
| Hematology | 51 (68.0) |
| Internal medicine ward No. 1 | 50 (66.7) |
| Internal medicine ward No. 2 | 36 (48.0) |
| Total | 384 (73.1) |
Prevalence of Antibiotic Use in Studied Patients Admitted to Ali-Ibn-E-Abitaleb (Peace Be Upon Him) Hospital (N = 525) by Hospital Ward in Zahedan, Iran, Within 2017 - 2018
The total antibiotic consumption defined by DBD in this study was 85.9 (data not shown). The most commonly used antibiotics, presented as DBD, in the obstetrics and gynecology, surgery, internal medicine, and hematology wards were clindamycin (15.2), cephalosporins (37.1), ciprofloxacin (28.4), and cotrimoxazole (221.7), respectively. The least frequently administered antibiotic in this study was cefixime (0.1).
Regarding individual antibiotic use, cotrimoxazole was the most commonly prescribed antibiotic with a DBD of 13.4, followed by metronidazole (10.3 DBD) and ceftazidime (9.8 DBD) (Table 2). Cephalosporins (18.7 DBD), sulfonamides (13.4 DBD), and imidazole derivatives (10.3 DBD) were the three antibiotics classes that ranked first among all administered antibiotics.
| Ranking | Antibiotics | ATC Code | DDD/100 Bed-Days |
|---|---|---|---|
| 1 | Cotrimoxazole | J01EE01 | 13.4 |
| 2 | Metronidazole | J01XD01 | 10.3 |
| 3 | Ceftazidime | J01DD02 | 9.8 |
| 4 | Clindamycin | J01FF01 | 7.9 |
| 5 | Ciprofloxacin | J01MA02 | 6.9 |
| 6 | Ceftriaxone | J01DD04 | 5.6 |
| 7 | Vancomycin | J01XA01 | 5.5 |
| 8 | Meropenem | J01DH02 | 5.1 |
| 9 | Cloxacillin | J01CF02 | 3.1 |
| 10 | Piperactam | J01CR05 | 2.9 |
The 10 Most Commonly Prescribed (Top 10) Antibiotics Expressed as Defined Daily Dose/100 Bed-Days in Patients Admitted to Ali-Ibn-E-Abitaleb (Peace Be Upon Him) Hospital in Zahedan, Iran, Within 2017 - 2018
Table 3 shows the comparison of the average of one year of antibiotics use (DBD) in surgical and nonsurgical wards. The antibiotic use was calculated for each ward monthly. For a better comparison, internal medicine and surgery wards were grouped into single groups. No statistically significant difference was observed in antibiotic use between different wards.
Comparison of Average of 12 Months Use of Antibiotics (Defined Daily Dose/100 Bed-Days) in Surgical and Nonsurgical Wards in Patients Admitted to Ali-Ibn-E-Abitaleb (Peace Be Upon Him) Hospital in Zahedan, Iran, Within 2017 - 2018 a
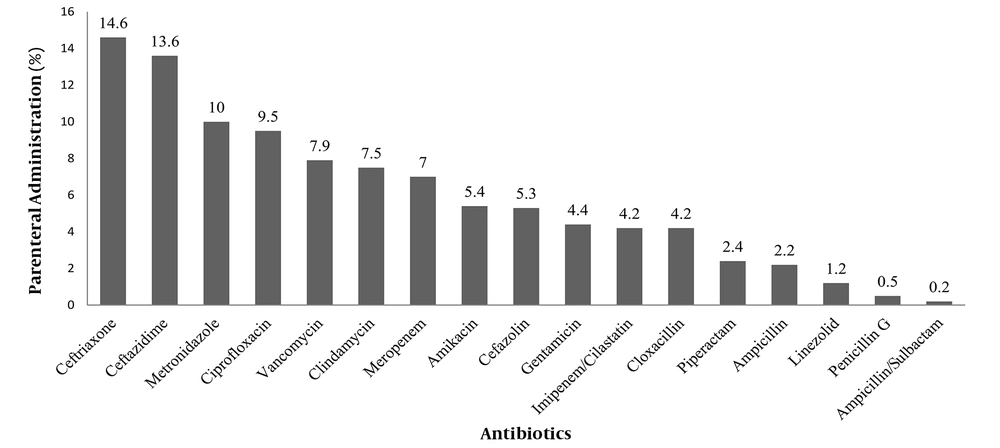
Overall, 68% (261 out of 384) of the studied patients received parenteral antibiotic therapy. In this study, the patients received a total of 12,630 doses of antibacterial agents, out of which 23.5% (2975 doses) and 76.4% (9655 doses) were administered orally and parenterally, respectively. The five most common antibiotics that were parenterally administered included ceftriaxone, ceftazidime, metronidazole, ciprofloxacin, and vancomycin, respectively (Figure 1).
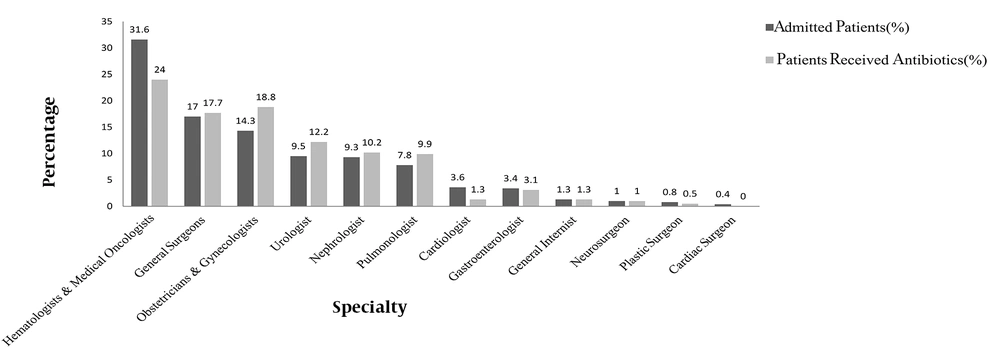
Out of 225 patients admitted to the surgical wards (ie, obstetrics and gynecology and men’s and women’s surgery), 173 patients underwent surgery, 74.6% and 25.4% of whom received antibiotics as prophylaxis and targeted treatment, respectively. The findings showed that a disproportionately higher proportion of patients were given antibiotic therapy by some specialties (Figure 2). The biggest difference between the percentage of hospitalized patients and the percentage of patients taking antibiotics was observed for obstetricians and gynecologists (4.5.3%), followed by urologists (2.7%) and pulmonologists (2.1%).
5. Discussion
This cross-sectional study was carried out in a tertiary care hospital in southeast Iran over a one-year period (2017 - 2018). The patterns of antimicrobial consumption were assessed using DBD as a standard measurement unit. In general, 73.1% of the studied patients received antibiotics, and the total antibiotic use in this hospital was 85.9 DBD. The most commonly used individual antibiotic was cotrimoxazole (13.4 DBD), and the most frequently prescribed antibiotic class was cephalosporins (18.7 DBD).
The percentage of patients receiving antibiotics in this study (73.1%) was higher than those reported in other Iranian hospitals (57% and 25.2%) (10, 11) and hospitals in countries, such as China (56%) (12), Turkey (54.4% and 47%) (13, 14), and France (19.5%) (15). However, the proportion was lower than those reported in a hospital in Iran (92.7%) (16) and Indonesia (84%) (17).
The utilization of antimicrobial agents in total was 85.9 DBD, which was lower than antibiotic use in Iranian hospitals, including hospitals in Tabriz (119.62) (18), Tehran (101.92) (10), and Sari (124) (19), but higher than the rate of Zanjan (79.79) (20).
The present study’s results showed that among patients undergoing surgery, 74.6% received antibiotics as prophylaxis, which is much higher than the rates reported in hospitals in Tehran (49.9%) (11) and Indonesia (15%) (17). The perioperative antibiotic prophylaxis is the standard of care and has been routinely used to prevent postoperative infectious complications. However, it seems that a striking fraction of this prophylaxis is inappropriate.
The highest proportion of patients with antibacterial therapy was among those admitted to the obstetrics and gynecology ward followed by surgical wards, and the lowest rate was reported in the patients of the internal medicine wards. The findings of the current study are consistent with reports from hospitals in Iran (10, 16, 19) and other countries, such as China (12) and Sri Lanka (21), which reported that the antibiotic prescription rate was the highest and lowest in surgery wards and medical wards, respectively.
The present study’s findings showed that a disproportionately higher percentage of patients treated by obstetricians and gynecologists followed by urologists and pulmonologists received antimicrobials. Antibiotic stewardship interventions targeting the antibiotic prescribing practices across specialties are needed to correctly diagnose infections and provide an indication for the prescribed antibiotics during the treatment course (22).
A wide range of cultural, contextual, and behavioral factors have been identified as the major determinants of antibiotic use at the country, hospital, and physician levels (23). At the country level, several factors, such as ideas about health, causes of disease, labeling of illness, coping strategies, and treatment modalities, play an important role in the determination of the antibiotic consumption pattern. At the hospital level, the pattern of antibiotic utilization is mainly influenced by organizational policies and the presence of a multi-professional care-delivery system (23). However, many of the observed variations in antimicrobial consumption patterns within and between hospitals are unlikely to be driven by differences in the epidemiology of infectious diseases or patient characteristics; nevertheless, it could mainly be explained by the prescriber’s behavioral factors (24).
5.1. Strengths and Limitations
One of the strengths of this study is that the ATC/DDD system was used as a standardized method to appropriately measure the usage of antibiotics. Moreover, the patient-level data about antibiotic consumption were collected for a period of one year. The data were collected by reviewing medical records and obtaining the information from the hospital HIS system. One of the limitations of the present study is that it was conducted at a single hospital, and the obtained findings cannot be generalized to hospitals in the province or country.
5.2. Conclusions
In conclusion, the findings of this study indicated that antibiotics, especially broad-spectrum agents, are commonly used in the inpatient setting. It is necessary to monitor antibiotic consumption data and implement evidence-based interventions to optimize antibiotic use in hospitals for the improvement of the quality of antibiotic prescription and lower bacterial resistance.