1. Context
The role of cardiovascular implantable electronic devices (CIEDs) in cardiac disease management has increasingly prominent in the last 50 years and they have considerably increased patient survival and quality of life. Cardiac pacemaker technology first made its appearance in the middle of the 20th century, followed by implantations shortly thereafter. Subsequent developments in cardiac pacemaker technology have resulted in the improvement of CIEDs used up to now (1, 2). Over time, the battery weight and the size of CIEDs have reduced simultaneously, leading to a remarkable extension of functional capabilities of the devices.
Although the design efficiency of CIEDs has enhanced owing to controlling infections and administering antibiotics intraoperatively, relevant infections appear to be an increasing problem, which can even lead to potentially life-threatening complications (1).
CIED infections represent a significant global health threat because of increasing implant rates, aging, and associated comorbidities in the CIED population. Patients with either undiagnosed or untreated CIED infection have been reported to exhibit high mortality rates (3).
2. Evidence Acquisition
2.1. Selection of Relevant Studies
All criteria for inclusion/exclusion of studies were specified before the literature search. Eligible studies for the systematic review included clinical trials, observational cohort studies, case-control studies, case series, or case reports of CIED infections. Reviews, abstracts, letters to editor, and cross-sectional studies were also eligible if their information had not been published in any other form.
2.2. Search Strategy
To identify eligible studies, the search was conducted in electronic databases including MEDLINE, EMBASE, and Cochrane Central Register of Controlled Trials (CENTRAL) from inception to May 2017, using the following terms: cardiovascular implantable electronic devices, permanent pacemakers, infection, complication, diagnosis, treatment.
A manual search was concluded by the perusal of the reference lists of all relevant trials/reviews and contacts were made with experts on the subject as efforts to identify relevant unpublished data. Two authors with expertise in conducting systematic reviews completed independently the search and screening of titles and abstracts.
3. Results
3.1. Incidence and Epidemiology
The increase in the number of CIED infection-related hospitalizations is proportional to the number of cardiovascular implantations performed. Furthermore, hospital fatalities are reported to have doubled due to CIED infection (1).
3.2. Risk Factors
The most important factors associated with the increased risk of CIED infections include re-intervention for any reason and the increased number of leads being implanted (3-5). Renal dysfunction is reported as another important risk factor related to the ingestion of anticoagulants. Factors affecting CIED infections can be summarized as follows: (1) comorbidities in patients, (2) immunosuppression (renal dysfunction and corticosteroid use), (3) use of oral anticoagulants, (4) intraoperative aspects and neglecting to provide prophylactic antibiotics intraoperatively, (5) CIED replacement or revision, (6) the amount of indwelling hardware, (7) operator experience, and (8) fever within the 24-h period before implantation (1). The fatality rate can increase from 5 to 29% if bacteremia or endocarditis occurs as a result of improper CIED infection treatment.
3.3. Microbiology
The majority of the CIED infections are caused by staphylococcal species (1, 6-15) as identified in 8 out of 10 patients. A wide range of coagulase-negative staphylococci (CoNS) has been found to cause such infections and has been accredited to be a source of bacteriological specimen contamination. The frequent separation of CoNS with exact antibiotic susceptibility patterns supports their role as pathogens in CIED infections. Moreover, sometimes, in the case of polymicrobial infection, there are more than one species of CoNS involved (10, 11, 16). The presence of staphylococcal species resistant to oxacillin should be taken into consideration regarding empirical administration of antibiotics. Corynebacterium strains, Propionibacterium acnes, Gram-negative bacilli (8, 13) including Pseudomonas aeruginosa (17), and Candida strains have been described as minor pathogens of CIED infections. Pathogens such as nontuberculous mycobacteria (18, 19) and fungi other than Candida (20) have rarely been reported to cause CIED infections (20). There are various ways of acquisition of microorganisms causing CIED infections. In the majority of cases, the pathogens may be transmitted either from the patient’s skin or from inanimate objects or staff in the hospital.
3.4. Diagnosis
CIED infection may present with symptomatology mimicking various conditions. Most common signs are inflammatory changes in the generator pocket area with evidence of dehiscence of the infected skin with percutaneous exposure to the generator and sometimes even its leads. Such topical changes combined with soreness or discomfort often cause patients to visit their physicians. Fever and systemic toxicity signs and symptoms are rarely present in some patients including malaise, fatigue, anorexia, or reduced functional ability. The infection of CIED is sometimes the underlying cause in patients suffering from pyrexia of undetermined origin in whom no inflammatory alterations may exist in the CIED-pocket site. Before the administration of antimicrobial therapy, at least four blood cultures need to be drawn from patients who are suspected to have CIED infection. The manifestation of systemic toxicity or peripheral leukocytosis may not appear in some patients with bloodstream infection. Positive blood cultures for staphylococcal species may imply CIED infections. It is necessary to inform patients with CIED to consult cardiologists or medical specialists regarding CIED-related infectious diseases manifesting as fever or bloodstream infection of unknown origin. Clinically, patients need to be scrutinized for CIED infection in the cases of changes in the pocket in terms of color, volume, and skin adherence. Any attempt to drain the pocket should be avoided, as this could result in infection of the device. Up until recently, it had not been established whether skin lesions of the pacemaker pocket are associated with infections. Nevertheless, some studies illustrated that local signs of inflammation were related to infection in at least 75% of the patients. The remaining cases prove that bacterial colonization must be considered as a possibility. The localized infection of the CIED pocket is usually not accompanied by any systemic signs of infection. Undoubtedly, symptoms such as chills, fever, and general malaise are helpful in early diagnosis of the general infective process in the bloodstream.
At the first stages of CIED infection, laboratory tests including white blood cell count, erythrocyte sedimentation rate, and C-reactive protein could remain within the normal range even though the culture of leads extracted from the patients usually proves positive in 88% of the cases (12).
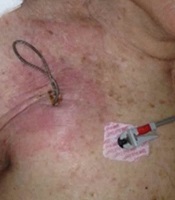
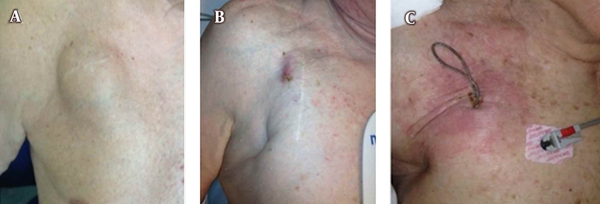
Transoesophageal echocardiography (TEE) should be carried out in patients who are suspected to infection or those with confirmed infection, as this is the most effective method in determining the presence of bacteria in valvular vegetations or vegetations along the leads (21, 22). TEE remains the ideal technique for the evaluation of vegetation size that is important to determine the optimal method of removing the infected leads (23). Table 1 illustrates the American Heart Association guidelines on the diagnosis of CIED infections. Time is of the essence in reaching a diagnosis and subsequently treating patients with possible CIED infection; delayed diagnosis could otherwise affect the patient’s prognosis. Figure 1 shows some cases with diagnosed pocket infection of CIED in our clinics.
| Recommendations for Diagnosis of CIED Infection and Associated Complicationsa | |
|---|---|
| Class I | 1. All patients should have at least two sets of blood cultures drawn at the initial evaluation before the prompt initiation of antimicrobial therapy for CIED infection (level of evidence: C). |
| 2. Generator-pocket tissue Gram’s stain and culture and lead-tip culture should be obtained when the CIED is explanted (level of evidence: C). | |
| 3. Patients with suspected CIED infection who have either positive or negative blood cultures but have had recent antimicrobial therapy before obtaining blood cultures should undergo TEE for CIED infection or valvular endocarditis (level of evidence: C). | |
| 4. All adults suspected of having CIED-related endocarditis should undergo TEE to evaluate the left-sided heart valves even if transthoracic views demonstrate lead-adherent masses. In pediatric patients with good views, transthoracic echocardiography may be sufficient (level of evidence: B). | |
| Class IIa | 1. Patients should seek evaluation for CIED infection by cardiologists or infectious disease specialists if they develop fever or bloodstream infection for which there is no initial explanation (level of evidence: C). |
| Class III | 1. Percutaneous aspiration of the generator pocket should not be performed as part of the diagnostic evaluation of CIED infection (level of evidence: C). |
| Recommendations for Antimicrobial Management of CIED Infection 1 | |
| Class I | 1. The choice of antimicrobial therapy should be based on the identification and in vitro susceptibility results of the infecting pathogen (level of evidence: B). |
| 2. The duration of antimicrobial therapy should be 10 to 14 days after CIED removal for pocket-site infection (level of evidence: C). | |
| 3. The duration of antimicrobial therapy should be at least 14 days after CIED removal for bloodstream infection (level of evidence: C). | |
| 4. The duration of antimicrobial therapy should be at least four to six weeks for complicated infection (i.e., endocarditis, septic thrombophlebitis, or osteomyelitis or if bloodstream infection persists despite device removal and appropriate initial antimicrobial therapy (level of evidence: C). | |
| Recommendations for Removal of Infected CIED | |
| Class I | 1. Complete device and lead removal is recommended for patients with definite CIED infection, as evidenced by valvular and/or lead endocarditis or sepsis (level of evidence: A). |
| 2. Complete device and lead removal is recommended for all patients with CIED pocket infection as evidenced by abscess formation, device erosion, skin adherence, or chronic draining sinus without clinically evident involvement of the transvenous portion of the lead system (level of evidence: B). | |
| 3. Complete device and lead removal is recommended for patients with valvular endocarditis without definite involvement of the lead(s) and/or the device (level of evidence: B). | |
| 4. Complete device and lead removal is recommended for patients with occult staphylococcal bacteremia (level of evidence: B). | |
| Class IIa | 1. Complete device and lead removal is reasonable in patients with persistent occult Gram-negative bacteremia despite appropriate antibiotic therapy (level of evidence: B). |
| Class III | 1. CIED removal is not indicated for a superficial or incisional infection without the involvement of the device and/or leads (level of evidence: C). |
| 2. CIED removal is not indicated for relapsing bloodstream infection due to sources other than CIED but long-term suppressive antimicrobials are required. (level of evidence: C) | |
| Recommendations for New CIED Implantation After Removal of Infected CIED1 | |
| Class I | 1. Each patient should be evaluated carefully to determine whether there is a continued need for a new CIED (level of evidence: C). |
| 2. The replacement device implantation should not be ipsilateral to the extraction site. Preferred alternative locations include the contralateral side, the iliac vein, and epicardial implantation (level of evidence: C). | |
| Class IIa | 1. When blood cultures were positive before extraction, blood cultures should be drawn again after device removal and should be negative for at least 72 hours before new device placement is performed (level of evidence: C). |
| 2. New transvenous lead placement should be delayed for at least 14 days following CIED system removal when there is evidence of valvular infection (level of evidence: C). | |
American Heart Association Recommendations for Diagnosis, Treatment, Removal, and Reimplantation of CIED
Various presentations of pocket infection of implantable cardiovascular electronic devices. A, voluminous collection inside the generator pocket in a patient presenting fever. B, pus and erythematous skin overlying the subcutaneous route of a lead. C, dehiscence of infected skin with percutaneous exposure of the generator and leads.
3.5. Treatment
In the case of CIED infection situated near the surface of the pocket site without the direct involvement of the device, removal is not deemed necessary. Nevertheless, oral administration of antibiotics with antistaphylococcal activity is suggested for 7 to 10 days. In patients with infected CIED, beside adjuvant therapy with antimicrobials, the immediate removal of the device should be performed regardless of the previous administration of antimicrobials. The proper antimicrobial agent should be chosen based on identification tests and in vitro susceptibility examination results. As the majority of infections are due to staphylococcal species, a proportion of which is resistant to oxacillin, the empirical administration of vancomycin should start to ensure antibiotic coverage until lab culture results are available. Vancomycin ought to stop for patients with oxacillin-susceptible staphylococcal strain infections and treatment with cefazolin or nafcillin should initiate. Vancomycin should continue for patients who are not candidates for b-lactam antibiotic therapy or whose CIEDs are infected by oxacillin-resistant staphylococci. Pathogen identification and in vitro susceptibility testing should be taken into account to determine the appropriate treatment for the small number of patients with non-staphylococcal CIED infections. There are no clinical trial data to date to determine the period of antimicrobial therapy or indicate the time that the subsequent oral medication should initiate following complete device removal. Influential factors to be taken into consideration regarding treatment include the degree of infection, the pathogens involved, the presence of septicemia and its duration, and relevant consequences including the involvement of the heart valves, septic thrombophlebitis, or osteomyelitis. Following device removal, blood cultures need to be repeated to exclude sepsis.
If the CIED infection is restricted to the pocket site, the recommended duration of the antimicrobial therapy is from a week to a week and a half. Should the device manifest erosion with no sign of inflammatory change, treatment needs to continue for 10 to 14 days. When the results of susceptibility tests are obtained, the infected CIED must be removed and an oral regimen can be prescribed. Parenteral therapy should be administered for a minimum of two weeks following the extraction of the infected CIED for patients diagnosed with bloodstream infection. Regardless of whether TEE is negative for endocarditis in patients with positive blood cultures at least 24 hours following CIED removal, proper parenteral antimicrobial therapy should be administered for a minimum of four weeks. Before the placement of the new device, it is imperative to determine whether sufficient debridement and infection control are applied to all sites including both the generator site and the regional electrodes. The contralateral side is preferable for the placement of a new device (1). When needed, the replacement device implantation should be carried out in an alternative location such as the iliac vein or epicardial implantation (Table 1).
For patients with confirmed CIED infection, it is recommended to undergo complete removal of the entire system irrespective of location (subcutaneous, transvenous, or epicardial) (8, 13, 24). The same procedure must be followed for patients with a localized pocket infection despite the lack of clinical signs of systematic infection. Immediate complete CIED removal is required because the odds of infection reoccurrence are high owing to the retained hardware. CIED erosion in any section is an implication of contamination to the whole system and the intravascular lead portion, both of which must be removed immediately. Conservative treatment using only antibiotics is not effective in CIED infection. To cure the patient, the complete pacing/defibrillation system must be removed (3) (Table 1).
As mentioned before, patients with confirmed CIED infection are required to undergo complete hardware removal (device and lead extraction) irrespective of location (8, 13, 24) to avoid the reoccurrence of infection owing to the retained hardware. Taking into account the risks involved in the surgical removal of CIED, the percutaneous lead extraction method is preferable. Conventional transvenous lead extraction with standard guidewires can be used; however, advanced systems such as “locking system” and “outer sheaths” have been used over the past few years, providing safety and minimum complications. Nevertheless, percutaneous lead extraction is subject to risk and procedural difficulties may arise depending on the lead type and features. The removal of coronary sinus leads is much less difficult using simple manual traction as opposed to the removal of ICD leads that are more difficult to extract due to adhesions (25-27). Transvenous lead extraction should only be carried out by skilled medical teams who have accumulated a procedural volume quota to ensure the maintenance of their skills and be in a position to offer cardiothoracic surgery backup in the case of emergency thoracotomy or sternotomy (28, 29). Upon extraction, vegetation displacement may cause pulmonary embolism, especially when vegetations are large (30, 31). Although such cases with large vegetations are often asymptomatic, percutaneous extraction is still the preferred method as the systematic risk would be greater when surgical extraction is employed.
For patients who require extraction of leads with vegetations of less than 2 cm in diameter, the percutaneous method is preferred. It has been proposed that patients with large vegetations (more than 2 cm in diameter) should undergo surgery (32); however, for such patients, until further evidence is made available, each case ought to be examined before the decision of removing the leads either percutaneously or surgically. Lead removal should be carried out in cases that require contemporary valve replacement or infective endocarditis repair or for those with retained hardware following failed efforts of its percutaneous removal. Of note, elderly patients with co-morbidities face high mortality rates during surgical removal (10, 33-35).
Following the removal of CIED, it should be decided whether there is a need for reimplantation, which is deemed unnecessary in the majority of cases (13, 22). A one-year follow-up study carried out by Marijon et al. (36) showed that low-risk patients did not require a new device, as these patients had a spontaneous heart rate of higher than 45 bpm, no symptomatic asystole when monitored, a QRS duration of less than 120 ms when there was the mention of AV block history, and no signs of high-degree AV block when monitored continuously. These patients remained device-free contrary to those cases that manifested arrhythmias indicating the need for reimplantation (36).
The contralateral side is preferred for the localization of reimplantation (1). When feasible, replacement device implantation should be carried out in an alternative location such as the iliac vein or epicardial implantation (Table 1). It is unclear as to when reimplantation should be carried out. Factors that should be taken into consideration include dependence on pacemakers and implantable cardioverter defibrillators, sustained bacteremia, and persistent vegetation.
To avoid the risk of infection, reimplantation should not be done immediately (13, 22, 28, 37). There must be a minimum of 72 hours of negative blood cultures before scheduling any reimplantation. Should there be any indication of remnant valvular infection, there must be a 14-day waiting period before new implantation (13, 38).
Patients dependent on pacemakers and implantable cardioverter defibrillators require special treatment. The ipsilateral implantation of a temporary active-fixation RV lead attached to an externalized pacemaker coupled with administering antibiotics has been described as bridging therapy before the reimplantation of a permanent pacemaker in pacemaker-dependent patients with CIED infection (34, 35). This procedure allows for faster mobilization with a reduced risk of pacing-related adverse effects (34, 35, 39). However, temporary pacing should be avoided as bridging therapy, if possible, because it is a risk factor for subsequent cardiac device infections (30).
There have been incidents showing that pacemaker-dependent patients with CIED infection could obtain a subcutaneous leadless pacemaker before the extraction of the infected system. A study carried out by Kypta et al. involved six pacemaker-dependent patients with CIED infection, all of whom were subjected to lead extraction due to serious device infection. Half of these patients displayed inflammatory changes in the generator pocket area only, while the remaining patients suffered from both pocket and lead infection. The procedure involved the positioning of a temporary pacemaker in four patients 2 to 48 hours following lead extraction. The other two patients received a transcatheter pacing system (MicraTM) just before the removal of their transvenous pacemaker. No evidence of infection was reported in these six patients over the next three months, after which positron emission tomography was used to confirm that the subcutaneous leadless pacemaker was not infected (40). It should be noted that the subcutaneous leadless pacemaker can be implanted on the condition that only single ventricular stimulation is required.
Patients with ICD-dependency and CIED infection can be treated with a wearable cardioverter defibrillator (WCD) as a “bridge” before ICD implantation. A German register has shown that 12% of all wearable cardioverter-defibrillator patients in Germany used the WCD as bridging therapy following ICD explantation due to CIED infection (41). The subcutaneous ICD can be an option used in place of the traditional ICD system in cases with limited venous accessibility following the extraction of infected ICD, provided that antibradycardia or antitachycardia pacing is not required (42).
Patients who suffer from CIED infections and cannot be subjected to device removal using percutaneous or surgical methods must be given long-term antimicrobial suppressive therapy (3). Such cases are those who have been given a short-term life expectancy or do not accept device removal. For long-term suppressive therapy, certain criteria must be met including stable cardiovascular status, improved clinical status with initial antimicrobial therapy along with no evidence of bloodstream infection. Due to the non-existence of relevant trials, the type of antimicrobial therapy and dosing schemas are yet to be defined. Numerous CIED infections induced by multidrug-resistant pathogens that are manifested in healthcare facilities can limit treatment options. Therefore, oral antimicrobial therapy is insufficient to suppress prolonged infection (1).
4. Conclusions
CIED infection poses a very serious threat. Early diagnosis is crucial to the survival of patients. The use of antibiotics and conservative approach without the CIED system removal is insufficient to properly treat this condition. Therefore, available guidelines suggest the removal of CIED and lead extraction. For those patients wrongly diagnosed and treated conservatively, a mortality rate of up to 17% within a year is reported. Thus, mortality rates could be greatly reduced via prompt diagnosis and CIED removal. At centers with highly experienced medical staff, procedures involving device removal and endovascular lead extraction are extremely effective and safe with perioperative mortality rates of lower than 1%.
Some innovative studies have shown that pacemaker-dependent patients with CIED infection could, under certain conditions, obtain a subcutaneous leadless pacemaker instead of a conventional transvenous pacemaker before the extraction of the infected system. Furthermore, patients dependent on implantable cardioverter defibrillators may obtain a subcutaneous ICD instead of a traditional ICD system before the removal of the infected system.