1. Background
Intestinal parasitic infections (IPIs) are among groups of parasitic infectious diseases that constitute a major public health problem globally, belonging to the class nematodes and protozoans (1). It is a condition in which parasites predominantly infect the gastrointestinal tract of humans, residing particularly in the intestinal wall (2). In 2012, the World Health Organization (WHO) estimated that 270 million preschool-aged children and over 600 million school-aged children lived in areas where helminths and intestinal protozoa are intensively transmitted and thus warrant interventions (3).
Intestinal parasitic infections are chronic infections that can have detrimental effects, particularly in children, such as trauma, nutrition-robbing and poisoning, changes in resistance, and immune suppression (1, 4). Multiple and interactive mechanisms can cause or aggravate anemia caused by intestinal parasites (5, 6). Hookworms, schistosomiasis, and Trichuris trichiura lead to intestinal blood loss and inflammation-induced restriction of iron absorption (5, 6). Many intestinal parasites reduce appetite and compromise nutrient absorption. Further helminthiasis-induced intestinal inflammation may limit nutrient absorption. By causing intestinal mucosal damage, impairing digestion, or causing diarrhea, intestinal parasites may cause indigenous nutrient loss. Additionally, Giardia lamblia and Entamoeba histolytica can also cause blood loss.
Intestinal parasitic infections adversely affect the nutritional status of children under five years (7). The most common intestinal parasite infections are caused by Ancylostoma lumbricoides, Trichuris trichiura, Hookworm, Strongyloides stercoralis, Enterobius vermicularis, and Toxocara species. S.stercoralis and Hookworm are transmitted directly through the skin, while the rest are transmitted through the mouth (8, 9).
Using contaminated household items, eating contaminated food, or eating with contaminated hands facilitates oral-route transmission. It has also been suggested that houseflies (Musca domestica) contribute to household items and food contamination (10).
Due to their vulnerability, intestinal parasites are more prevalent among primary school children in Nigeria and are the leading cause of morbidity and mortality among primary school children. Intestinal parasitic infections have been reported to have a high prevalence among children in Nigeria because of their vulnerability (11-14). Nevertheless, information regarding their current status is limited, particularly in BAYELSA, a region of the Niger Delta in Nigeria.
2. Objectives
This study examined intestinal parasitic infections (IPIs) and associated risk factors in primary school-aged children (5 - 15 years old) in various communities in Sagbama Local Government Area, Bayelsa State, Nigeria.
3. Methods
3.1. Study Areas
The study was carried out in nine riverine communities:
- Adagbabiri (5°12’6.246” N, 6°12’26.928”E),
- Angalabiri (5°5’30.209” N, 6°2’18.420”E),
- Bulou-Orua (5°8’20.850” N, 6°4’54.228”E),
- Ebedebiri (5°8’20.850” N, 6°4’54.228”E),
- Ofoni (5°6’40.124” N, 6°3’21.237”E),
- Sagbama (5°9’11.422” N, 6°11’45.744”E),
- Toru-Angiama (5°8’23.166” N, 6°6’27.558”E),
- Toru-Orua (5°6’5.346” N, 6°3’51.588”E),
- Trofani (5°18’6.565” N, 6°19’40.632”E).
All in Sagbama Local Government Area, Bayelsa State, Nigeria. Communities in this area are generally rural. Homes are built in block houses, clustered homesteads of mainly mud houses enclosed by bamboo sticks. Sagbama's climate and vegetation are consistent with that of a typical rainforest region in Southern Nigeria. The basic occupation of the inhabitants includes farming, fishing, and hunting. Some are self-employed, traders, commercial boat and vehicle drivers/transporters, and a few are public servants, civil servants, and retired civil servants. The villages are located on a coastal plain with many ponds, streams, and rivers. The state of Bayelsa experiences heavy rainfall from May to October, with the peak occurring in August. Most of these communities are affected by flooding due to the rainforest's vegetative cover. The dry season begins in November and ends in April. The average temperature in the area ranges from 25 to 34 degrees Celsius. The crops grown in these communities include sugarcane, banana, plantain, cassava, yam, beans, garden egg, fresh tomatoes, fresh pepper, cucumber, groundnut, Okro, banana, cocoyam, water yam, and vegetables which are planted and cultivated in large quantity basically for consumption and commercial purposes. Most of the inhabitants in these communities use water from the Forcados River as a local source of drinking water for waste disposal, fecal disposal, and other domestic activities. Inhabitants also use the community taps and individual boreholes as drinking water sources, which only flow at a certain period of the day. There are toilet facilities, but most people use the surrounding bushes, ponds, and streams as toilets.
3.2. Consent and Approval
The Department of Public Health, the State Ministry of Health, and the State Primary Schools Board provided ethical approval. Written informed consent was obtained from the Community Development Committee (CDC), and informed consent was obtained from the community chiefs and elders before the study. The parents, teachers, and participants were properly enlightened on the study's aims, objectives, benefits, and protocols, the need for voluntary participation, and the right to stop participation at any time.
3.3. Sample Collection
A total of 622 stool samples were collected from primary school children between the ages of 5 - 15 years using clean 50 cm3 wide-mouthed, screw-capped universal specimen bottles. All samples were collected using structured questionnaires that asked for basic epidemiological information. This information included their ages, sexes, classes, parents' occupations, children's hygiene, histories of their contact patterns, and activities with water bodies. The specimens were labeled appropriately on the submission of stool samples and properly corked. On-the-spot macroscopic analysis of motile trophozoites was conducted using a direct technique. For examination and further analysis, the samples were placed in a cooling container and taken to the Parasitology Research Laboratory of the Department of Animal and Environmental Biology, University of Port Harcourt.
3.4. Stool Sample Analysis
The samples were analyzed by direct wet mount and formal ether concentration method as described by Cheesbrough (15).
3.5. Statistical Analysis
All data collected were analyzed using frequency tables and graphic representations of bar charts using the SPSS version 9.3 (SAS Institute, Inc., Cary, NC, USA). To test whether the infection was statistically significant at 0.05 alpha levels across the population, analysis of variance (ANOVA) was employed. A post hoc test using Tukey's HSD (honest significant difference) demonstrated prevalence across communities.
4. Results
A total of 149 (23.95%) school children tested positive for intestinal parasitic infections. Nine species of intestinal parasites were encountered, namely; Ascaris lumbricoides 49 (7.88%), Entamoeba histolytica 31 (4.98%), Strongyloides stercoralis 13 (2.09%), Giardia lamblia 12 (1.93%), Hookworm 11 (1.77%), Trichuris trichiura 11 (1.61%), Schistosoma mansoni (1.45%), Diphyllobothrium latum 4 (0.64%) and Fasciola hepatica 2 (0.32%) in this order (Table 1). In terms of communities, Adagbabiri was observed to have the highest prevalence in the study, recording 11 (47.83). Afterwards, Sagbama recorded 48 (37.5%), Trofani recorded 12 (32.43%), Toru-Orua recorded 14 (27.50%), Angalabiri recorded 21 (19.81%), Ebedebiri recorded 14 (18.92%), Toru-Angiama recorded 6 (16.22%), Bulou-Orua recorded 20 (15.04%), and Ofoni recorded 3 (9.09%) in this order. There was a significant difference in prevalence between the nine communities studied at P < 0.05 (P = 0.001). The prevalence in the Adagbabiri community was not significantly different from that in the other communities at P > 0.05, except for Sagbama (P = 0.014). The Angalabiri and Buluo-Orua communities did not show any significant differences from other communities. Edebebiri showed no significant difference from other communities except Sagbama (P = 0.018). Additionally, the prevalence in the Ofoni community was significantly different from the Sagbama community (P = 0.000), but it did not differ significantly from other communities. The study found that the highest prevalence of intestinal parasitic infections occurred in ages 5 - 7 years 59 (30.26%). After this, ages 8 - 10 had 43 (26.53%), and ages 11 - 13 had 31 (22.92%). The lowest rate was recorded for age brackets 14 - 16, with 16 (12.60%) (Table 2). In these nine communities, intestinal parasitice infections were significantly different at P < 0.05 (P = 0.049) among the sampled population. Furthermore, there was a significant difference between the infected populations in each of the nine communities. Table 3 shows the sex-related infections among the nine communities. Out of the population examined, 84 (25.07%) males tested positive against 65 (22.65%) females. The population of infected males showed no significant difference from those of infected females at P < 0.05 (P = 0.647).
| Communities | No. Examined | No. Infected | Intestinal Parasites Species | Prevalence (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A. lumbricoides | E. Histolytica | Hookworm | G. lamblia | S. stercoralis | T. trichiura | D. latum | F. hepatica | S. mansoni | Mixed infection | ||||
| Adagbabiri | 23 | 11 | 3 | 3 | 0 | 0 | 3 | 1 | 0 | 0 | 1 | 3 | 47.83 |
| Angalabiri | 106 | 21 | 2 | 3 | 0 | 0 | 1 | 1 | 0 | 0 | 2 | 4 | 19.81 |
| Bulou-Orua | 133 | 20 | 7 | 3 | 2 | 5 | 1 | 1 | 0 | 0 | 1 | 6 | 15.04 |
| Ebedebiri | 74 | 14 | 3 | 3 | 3 | 0 | 1 | 2 | 1 | 1 | 0 | 2 | 18.92 |
| Ofoni | 33 | 3 | 2 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 9.09 |
| Sagbama | 128 | 48 | 16 | 12 | 5 | 6 | 2 | 3 | 3 | 0 | 1 | 6 | 37.50 |
| Toru-Angiama | 37 | 6 | 3 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 16.22 |
| Toru-Orua | 51 | 14 | 3 | 2 | 0 | 0 | 4 | 1 | 0 | 1 | 3 | 3 | 27.50 |
| Trofani | 37 | 12 | 6 | 2 | 2 | 0 | 0 | 1 | 0 | 0 | 1 | 3 | 32.43 |
| Total | 622 | 149 | 45 | 31 | 11 | 12 | 13 | 10 | 4 | 2 | 9 | 28 | 23.95 |
| Age Groups in Years | Adagbabiri | Angalabiri | Bulou-Orua | Ebedebiri | Ofoni | Sagbama | Toru-Angiama | Toru-Orua | Ofoni | Total No. Examined | Total No. Infected | Prevalence (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N.E | N.I | N.E | N.I | N.E | N.I | N.E | N.I | N.E | N.I | N.E | N.I | N.E | N.I | N.E | N.I | N.E | N.I | ||||
| 5 - 7 | 8 | 4 | 34 | 10 | 45 | 9 | 19 | 4 | 11 | 2 | 44 | 16 | 9 | 3 | 13 | 5 | 12 | 6 | 195 | 59 | 30.26 |
| 8 - 10 | 5 | 3 | 29 | 6 | 35 | 5 | 24 | 7 | 8 | 1 | 28 | 12 | 11 | 1 | 19 | 5 | 3 | 3 | 162 | 43 | 26.53 |
| 11 - 13 | 3 | 2 | 17 | 3 | 23 | 3 | 17 | 3 | 9 | 0 | 32 | 14 | 13 | 2 | 10 | 3 | 11 | 1 | 135 | 31 | 22.92 |
| 14 - 16 | 7 | 2 | 26 | 2 | 30 | 3 | 14 | 0 | 5 | 0 | 24 | 6 | 4 | 0 | 9 | 1 | 8 | 2 | 127 | 16 | 12.6 |
| Total | 23 | 11 | 106 | 21 | 133 | 20 | 74 | 14 | 33 | 3 | 128 | 48 | 37 | 6 | 51 | 14 | 37 | 12 | 622 | 149 | 23.95 |
Abbrevitions: N.E, number examined; N.I, number infected.
| Communities | Gender | Prevalence (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total No. Examined | Total No. Infected | Total Prevalence of Males and Females (%) | Total No. of Males Examined | Total No. of Males Infected | Total No. of Females Examined | Total No. of Females Infected | Males | Females | |
| Adagbabiri | 23 | 11 | 47.83 | 15 | 8 | 8 | 3 | 53.33 | 37.50 |
| Angalabiri | 106 | 21 | 19.81 | 55 | 9 | 51 | 12 | 16.36 | 23.53 |
| Bulou-Orua | 133 | 20 | 15.04 | 76 | 11 | 57 | 9 | 14.47 | 15.79 |
| Ebedebiri | 74 | 14 | 18.92 | 48 | 5 | 26 | 9 | 10.42 | 16.01 |
| Ofoni | 33 | 3 | 9.09 | 16 | 2 | 17 | 1 | 12.50 | 5.88 |
| Sagbama | 128 | 48 | 37.50 | 73 | 30 | 55 | 18 | 41.10 | 32.73 |
| Toru-Angiama | 37 | 6 | 16.22 | 20 | 4 | 17 | 2 | 11.76 | 11.76 |
| Toru-Orua | 51 | 14 | 53.85 | 14 | 9 | 37 | 5 | 75.00 | 13.15 |
| Trofani | 37 | 12 | 32.43 | 18 | 6 | 19 | 6 | 33.33 | 31.58 |
| Total | 622 | 149 | 24.96 | 335 | 84 | 287 | 65 | 25.07 | 22.65 |
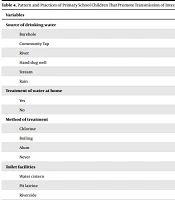
The source of drinking water, toilet facilities, washing of hands after using the restroom, washing of hands before meals, waste disposal pattern, and washing of hands following waste disposal were also found to be associated with intestinal parasitic infections (Table 4).
| Variables | No. Examined | No. Infected | Prevalence (%) |
|---|---|---|---|
| Source of drinking water | |||
| Borehole | 263 | 11 | 4.18 |
| Community Tap | 120 | 20 | 16.67 |
| River | 142 | 91 | 64.08 |
| Hand-dug well | - | - | - |
| Stream | - | - | - |
| Rain | 97 | 27 | 27.84 |
| Treatment of water at home | |||
| Yes | 370 | 22 | 5.95 |
| No | 252 | 127 | 50.40 |
| Method of treatment | |||
| Chlorine | 10 | - | - |
| Boiling | 30 | 2 | 6.67 |
| Alum | 330 | 21 | 6.36 |
| Never | 252 | 127 | 50.40 |
| Toilet facilities | |||
| Water cistern | 35 | 3 | 8.57 |
| Pit latrine | 175 | 34 | 19.43 |
| Riverside | 412 | 112 | 27.18 |
| The pattern of hand washing after using the toilet | |||
| Soap and water | 210 | 27 | 12.86 |
| Ash and water | 45 | 2 | 4.44 |
| Water alone | 339 | 95 | 28.02 |
| Waste disposal pattern | |||
| River/stream | 413 | 92 | 22.28 |
| Dust bin | 28 | 11 | 39.29 |
| Garbage pit | 150 | 37 | 24.67 |
| Burning | 31 | 9 | 29.03 |
| Washing hands after garbage/waste disposal | |||
| Yes | 168 | 15 | 8.92 |
| No | 454 | 134 | 29.52 |
| Hand washing before eating meals | |||
| Yes | 477 | 14 | 2.94 |
| No | 145 | 135 | 93.10 |
| The pattern of washing raw vegetables/fruits before eating | |||
| Always | 101 | 7 | 6.93 |
| Sometimes | 89 | 18 | 20.22 |
| Rarely | - | - | - |
| Whenever l remember | 432 | 124 | 28.70 |
5. Discussion
The prevalence of 23.95% recorded in this study is relatively high. The rate is higher than the 21.0% recorded among primary school children in Gokana and Kana Local Government Areas (LGAs) of Rivers State (16). It is, however, slightly lower than the 27.66% reported in Port Harcourt (17) and 52.0% reported in Ilie, Osun State (8). Location, sanitation, and level of awareness may contribute to this variation. All the communities sampled in Sagbama Local Government are rural, which agrees with the settings (8, 16) above, except when compared to Port Harcourt (17), an urban setting. The level of health awareness and sanitation in an urban setting is usually superior to that of a rural setting. Nevertheless, periodic dewormings, mass screenings, and awareness creation programs may be responsible for the variation observed because the provision of adequate sanitation is not always possible in resource-poor settings, particularly in rural areas.
It was established that Ascaris lumbricoides, Entamoeba histolytica, Strongyloides stercoralis, Giardia lamblia, Hookworm, Trichuris trichiura, Schistosoma mansoni, Diphyllobothrium latum, and Fasciola hepatica were the intestinal parasites identified. At 7.88%, A. lumbricoides was the most prevalent parasite. The findings of this study are consistent with those of earlier researchers (4, 8, 18, 19), who identified A. lumbricoides as the most prevalent and important helminth.
According to age-related prevalence, infection was most prevalent in those between the ages of 5 - 7 years, followed by those between the ages of 8 - 10 years, and those between the ages of 14 - 16 years. In this study, the number of parasitic infections decreased as the children's age increased. The results of this study are consistent with findings from the study of intestinal parasite prevalence in school-age children in Lafia Nassarawa State (20). This study found that male children had a higher infection rate than female children in most communities. This finding agrees with those of other researchers (16, 17) but differs from those of Tongiura et al. (20), who found a higher infection rate in females than in males. It may be because male children engage in outdoor activities, whereas female children mostly do household chores indoors.
Risk factors associated with the distribution and spread of intestinal parasites infection among school-age children showed that source of drinking water, toilet facilities, washing of hands after using the toilets, washing of hands before meals, the pattern of waste disposal, and washing of hands after waste disposal were associated with Intestinal parasitic infections. This is based on the findings of Gizaw et al. (21) which reaffirms WHO's position safe and sufficient water, sanitation, and hygiene (WASH) play a key role in preventing numerous neglected tropical diseases NTDs such as intestinal parasitic infections, soil-transmitted helminths, and schistosomiasis (22). Most children in the Sagbama local government area with a river as their source of drinking water were infected with intestinal parasites. Also, a greater percentage of those that never treated their water at home were more infected than those that treated their water which further buttresses the crucial role of WASH in the reduction of transmission of intestinal parasitic infections and soil-transmitted helminths. In the same vein, people who used Riverside as their toilet facilities were more infected than those who used other facilities such as water cistern and even pit as their toilet. Furthermore, higher infection rates were observed among those who didn't wash their hands before meals compared to those who did; and among those who didn't wash their hands after waste/garbage disposal compared to those who did. These observations further lay credence to the fact that increased access to improved water, sanitation, and hygiene (WASH) infrastructure and services contributes greatly to the reduction in the STH disease burden by reducing exposure to Soil-Transmitted Helminths STH infective stages in the environment (23).
5.1. Conclusions
Intestinal parasitic infection is relatively prevalent in the Sagbama local government area of Bayelsa State. Provision of safe drinking water, improved sanitation, and a step-up in health education in the communities or local government will reduce the exacerbation of intestinal parasitic infection in the Local Government Area.