1. Background
Leishmaniasis is one of the most important parasitic diseases worldwide. Cutaneous leishmaniasis (CL) is still a great health problem in Iran (1). Leishmaniasis includes a wide range of diseases that occur in different clinical forms, including cutaneous, visceral, muco-cutaneous, and mucosal leishmaniasis (2, 3). Nearly 0.7 to 1.3 million new cases of CL are registered in the world annually (3). Cutaneous leishmaniasis is the most common form of leishmaniasis, which causes skin lesions, especially sores, on various parts of the body, which in turn cause permanent wounds, severe disability, and scarring. About 95% of CL cases occur in the Americas, Mediterranean, Middle East, and Central Asia. In 2019, more than 87% of new CL cases occurred in 10 countries, including Afghanistan, Algeria, Brazil, Colombia, the Islamic Republic of Iran, Iraq, Libya, Pakistan, the Syrian Arab Republic, and Tunisia. It is estimated that between 600,000 and 1 million new cases occur worldwide each year (4). Almost all known centers of this disease are located between 2 latitudes of 28 to 42 degrees north (5).
Of the total annual cases of leishmaniasis in Iran, about 80% are related to rural cutaneous leishmaniasis, 5% to visceral leishmaniasis, and the rest to urban cutaneous leishmaniasis. This statistic is much lower than the actual rate for various reasons, including the lack of referral of a significant number of patients to the physicians; especially in deprived areas, various problems in diagnosing the disease, and low sensitivity of conventional diagnostic methods in laboratories (6). Leishmaniasis imposes a heavy economic burden on families, communities, and countries; developing countries, in particular. Glucantime is an expensive drug for treating leishmaniasis, and its application requires multiple visits and injections. Topical injection of the drug around the wound is also painful (7). Leishmaniasis skin lesions may take several months to get healed. Even with a successful treatment, scarring can cause psychological and emotional problems in the patient (8).
Numerous studies have shown that leishmaniasis is increasing in Iran as well as in the world. However, no definitive treatment has been developed for it and, therefore, physicians treat their patients based on their experiences, the acquired knowledge and instructions, and the application of pentavalent antimony from various drugs and methods. Results and reports suggest a growing trend in the number of patients (9).
2. Objectives
Taking into account the suitable conditions and many trips and immigration to Tehran province, this study aimed to investigate the epidemiological factors in patients with cutaneous leishmaniasis referred to health centers under the auspices of Shahid Beheshti University of Medical Sciences (SBMU) from 2011 to 2021 in order to improve prevention and treatment methods for high-risk individuals and provide appropriate health services for reducing the incidences of disease in high-risk areas.
3. Methods
The retrospective, descriptive-analytical study was conducted to investigate all individuals with cutaneous leishmaniasis between 2011 and 2021 who lived around the medical centers of SBMU and Health Services, referred to comprehensive health service centers, private offices, or public/private hospitals due to leishmaniasis-related skin lesions, and had medical histories registered in the portal system of the Ministry of Health.
Since many of the relevant portal options were required, no item was removed from the study. The collected data were analyzed using SPSS software version 26 after data entry. Tables and graphs were used to describe the data qualitatively, and the central indicators and dispersion were used to describe them quantitatively. Data analysis was performed using independent t-test and chi-square at a significance level of 5%.
4. Results
Out of 2119 patients referring to leishmaniasis treatment centers of SBMU during the studied years, 1382 ones were males (65.2%), and 737 ones were females (34.8%); 1877 patients (88.6%) lived in urban areas, and 238 ones (11.4%) lived in rural areas, and 4 patients were from unknown areas; 1734 ones were Iranians (81.8%), and 385 ones were Afghan citizens (18.31%), while 1884 patients (88.9%) had a history of traveling to other regions and 235 ones (11.1%) had not included their travel history in their submitted biographies. The mean age of the patients was 32.28 ± 20.38 years, and the median age was 30 years.
According to our study results, more than 51% of cases were common in the young age group less than 30 years, so that the 21 - 30 age group had the highest prevalence (18.4%) followed by children under 10 years (17.1%), the 40 age group (16.5%), the 11 - 20 age group (16%), and the 41 - 50 age group (11.6%); the lowest prevalence, on the other hand, was detected for the 51 - 60 age group (8.6%).
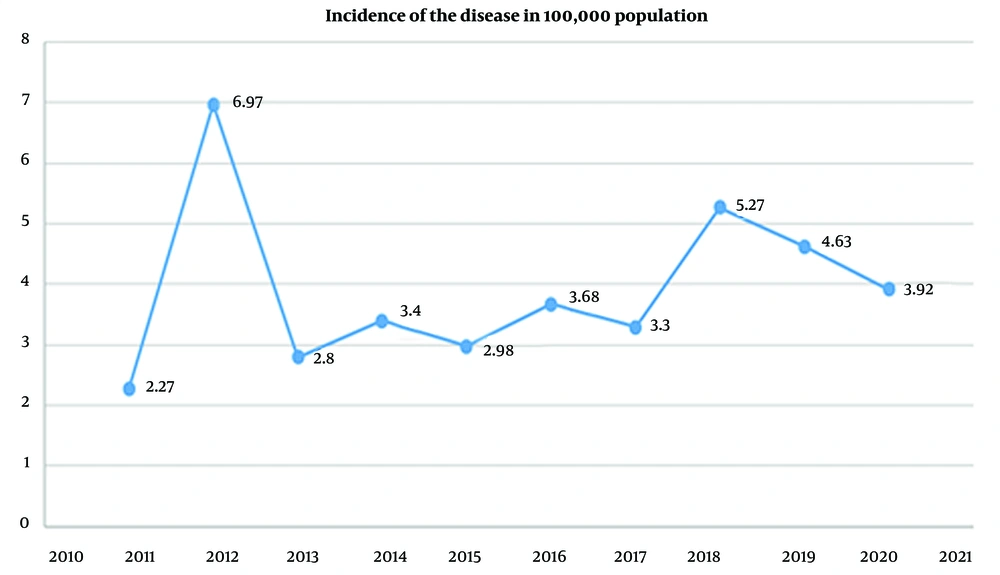
Based on the results of this study, from 2011 to 2018, the prevalence of cutaneous leishmaniasis has been increasing. In 2018, the number of identified patients (14.1%) was almost 3 times this amount in 2011 (5.4%), and since then, it has had a downward and declining trend. Figure 1 shows the prevalence rate per 100,000 population.
According to our study results, moreover, the highest monthly prevalence of the disease was recoded for November with 21.4% of cases, followed by December with 17.2% and October with 14.1%. The lowest monthly prevalence during the given years, on the other hand, were recorded for July (2.7%) and April (3.4%), respectively.
The highest prevalence of disease was found for autumn with52.7%, and the lowest one was detected for spring with 11.4%. There was no statistically significant relationship between seasonal incidence and travel history (P = 0.221) (Table 1).
| Season and History of Travel | No. (%) | P-Value a |
|---|---|---|
| Spring | 0.221 | |
| Yes | 214 (88.4) | |
| No | 28 (11.6) | |
| Summer | ||
| Yes | 238 (86.2) | |
| No | 38 (13.8) | |
| Fall | ||
| Yes | 1007 (90.2) | |
| No | 110 (9.8) | |
| Winter | ||
| Yes | 425 (87.8) | |
| No | 59 (12.2) |
a Chi-square
According to the results of the patients’ smear test, 1856 patients had positive results, 71 ones had negative results, and 192 ones had no results. As for those needing parasitic culture results, 50 were positive, 15 were negative, and parasite culture had not been performed for 2054 ones. As for those undergoing PCR test, 16 were positive, and 6 were negative; and PCR had not been performed for 2097 ones. The most visited travel destinations of patients were Isfahan (16.1%) followed by Khorasan, Semnan and Damghan with 10.6% and 0.9%, respectively; the least visited destination, on the other hand, was Ardabil (0.1%).
According to the results of the lesion site examination, the highest number of bites were observed in the hand area (16.1%), and the lowest ones were observed in the trunk area (0.2%). Wounds were also observed in the foot area (15.9%), face (12.3%), forearm (9.1%), arm (6.7%), neck (2.2%), and more than 3 other areas (combination of different organs) (35.6%).
Our results were indicative of a statistically significant relationship between patients’ nationality and travel history (P = 0.0001). Furthermore, a statistically significant relationship was observed between patients’ nationality and gender (P = 0.0001) (Table 2). There was also a statistically significant relationship between job and travel history (P = 0.0001) (Table 3).
| Nationality and Gender | No. (%) | P-Value a |
|---|---|---|
| Iranian | 0.001 | |
| Male | 1088 (51.34) | |
| Female | 646 (30.48) | |
| Afghan | 0.001 | |
| Male | 294 (13.87) | |
| Female | 91 (4.29) |
a Chi-square
| History of Travel | No. (%) | P-Value a |
|---|---|---|
| Nationality | ||
| Iranian | 0.001 | |
| + | 1564 (73.8) | |
| - | 170 (8.02) | |
| Afghan | 0.001 | |
| + | 320 (15.1) | |
| - | 64 (3.02) | |
| Employment | ||
| Employed | 0.001 | |
| + | 1808 (86.32) | |
| - | 226 (10.66) | |
| Unemployed | 0.001 | |
| + | 76 (3.58) | |
| - | 9 (0.42) | |
a Chi-square
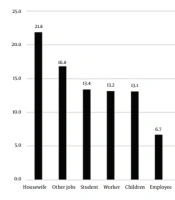
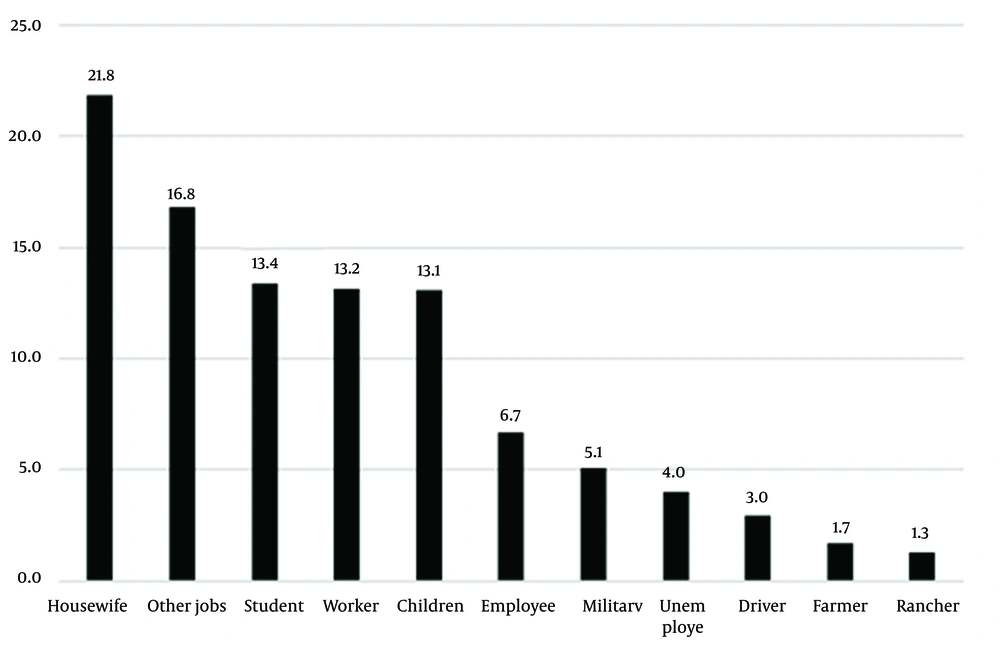
Figure 2 presents the occupations of patients during the studied years. Housewives showed the highest prevalence (21.8%), and ranchers showed the lowest (1.3%) prevalence. It seemed that the most common cause of cutaneous leishmaniasis in the study population was traveling to endemic parts of the country and bites by sandflies during travel (Table 3 and Figure 2). Travelers had traveled to local areas, including Kashan, Isfahan, Semnan and Garmsar, Khuzestan, Khorasan, Qom, and Golestan.
The most common parasite was Leishmania tropica (22.4%) followed by Leishmania major (16.4%), and 1296 parasites had not been identified except for Leishmania body. According to our results, the highest number of bites were observed in the hand area (16.1%), and the lowest number of bites were detected in the trunk area (0.2%).
5. Discussion
Out of 2119 patients referring to leishmaniasis treatment centers of Shahid Beheshti University of Medical Sciences during the studied years, the age mean was 32.28 ± 20.38 years, and the median age was 30 years.
Our study results were consistent with the findings from the following studies. In a study by Yaghoobi-Ershad et al., the highest rate for those with scars was 15.8% in the age group of 5 - 9 years, while the lowest rate was 2.0% in the age group of 0 - 4 years (1). Also, the prevalence of scars was 11.2% for those aged under 10 years, and it was 10.2% for those aged 10 years or older (1).
In the study by Alhawarat et al. in Jordan, more than 51% of the cases were from the young age group, and they had been exposed to this disease for less than 30 years, so that the age group of 21 - 30 years had the highest number of diseases (18.4%); and then among children under 10 years and the age group of the 40s and the second decade of age had more of such cases (10). Finally, the lowest number of diseases was recorded for those from the age group of 51 - 60 years (8.6%) (10).
In another study by Rassa et al. in Dasht-e Azadegan, which is considered as an endemic area of the disease, the highest number of diseases was detected for an age group smaller than the age group in our study; in the 10 - 19 age group (23.78%) and in the age group of 29 - 20 (23.63%) it was observed that 5% of the highest age group of our study was obtained (11). In the study by Amin et al. in Saudi Arabia, 50 percent of the cases were in the age group 15 - 45 years (12). In the study by Alawieh et al.’s in Lebanon, more than 70 percent of the cases were found to occur under the age of 15, which was not consistent with our study findings (13).
In the study by Alhawarat et al. in Jordan, 233 patients (19.13%) were under 5 years old, 451 ones (37.03%) were between 14 - 14 years old, 190 ones (15.60%) were between 15 - 24 years old, and 344 ones (28.24%) were between 25 - 30 years old. Of these, 646 (51.97%) were Jordanians and 559 (44.97%) were Syrians (10).
Seemingly, cutaneous leishmaniasis is most prevalent among teenagers and young adults aged 5 - 30, which can be due to their more engagement in outdoor activities. However, the prevalence of this disease among children is lower due to their longer stay at home and less exposure to bites.
According to the results of this study, 65.2% of patients were males, and 34.8% were females. There was a statistically significant relationship between the nationality of patients and gender. Our study results were consistent with findings of other studies in this regard. In the study by Rassa et al. in 2019, 54.54% of the patients were males and 44.46% were females (11). In a study by Mokhtari and Golmakani in Mashhad, Iran, 54% of patients were males and 46% were females (9). In another study by Alhawarat et al. in Jordan, a total of 1243 cases (60.65% male and 39.35% female) were diagnosed during the study period (10). A study by Lafta and Hussain in Iraq found no significant difference between age or gender (14). The results of the study by Özbilgin et al. in Turkey, where the number of men was higher than women (61% vs. 39%), were consistent with our study results (15). In the study by Abuzaid et al. in Saudi Arabia examining the incidences of cutaneous leishmaniasis in patients aged over 33 years, the incidence was 78% in men, whereas it was 22% in women (16).
In our study as well as in majority of the reviewed studies, the prevalence of leishmaniasis in men was higher than that in women, which may have been attributed to the presence of more men in high-risk areas home to the ground mosquito Phlebotomus papatasi. Also, men were more involved in outdoor activities such as farming than women.
Our study results demonstrated that 1877 patients (88.6%) lived in urban areas and 238 ones (11.4%) lived in rural areas, and 4 people were from unknown areas. Our study results were consistent with the findings of following studies. In the study by Rassa et al., 61.74% of the patients were from urban areas, and the remaining patients were from rural areas (11). In the study by Yaghoobi-Ershadi et al., Sandfly species collected from indoors and outdoors in Jovein rural district were reported to be 86.6 and 13.4%, respectively (1).
Moreover, 1734 of the patients were Iranians (81.8%) and 385 ones (18.1%) were Afghan citizens; and 1884 patients (88.9%) had a history of traveling to other regions, and 235 ones (11.1%) had not included their travel history. In the study by Alhawarat et al. in Jordan, no significant difference was discovered between Jordanians and Syrian refugees from 2010 to 2012 regarding the incidences of the disease (10).
According to the results of this study on patients referred to leishmaniasis treatment centers affiliated to SBMU from 2010 to 2020, there was an increasing trend from 2010 to 2018. In 2018, the number of identified patients was almost 3 times greater than the number of patients identified in 2010; however, this number followed a downward trend since then.
In the study by Eshetu and Mamo in north-central Ethiopia, the findings showed that the prevalence of cutaneous leishmaniasis was 5.1 per 100,000 population, which showed an increasing trend (17).
According to a report by Lafta and Hussain, the number of cases increased in Iraq since 2001 (14). The number of cases recorded in 2001 was 625 at a rate of 2.3 per 100,000, while this rate reached 45 per 100,000 in 2016; this figure, in effect, was 20 times higher than that in 2001. This increase in statistics may have been attributable to the developments in Iraq, which had been caused by the deterioration of public health due to adopting inappropriate preventive measures, lack of disease control, lack of access to specialized diagnosis and treatment, lack of medicine, poor environmental conditions, lack of control of sandflies and, more importantly, the displacement of millions of Iraqis as the result of internal unrest to nearly one-third of Iraqi territory leading to a 20-fold increase in the incidences of leishmaniasis since 2014 (14).
Philippe Desjeux from the World Health Organization identified a new set of 21st century health threats and their contributing factors about 20 years ago, including cutaneous, mucosal, and visceral leishmaniasis. The increase in mucosal leishmaniasis in modern world has been attributed to drought and deforestation, urbanization in Brazil and Venezuela, as well as human migration from mountainous areas to lowlands; while the increase in the incidence of leishmaniasis in the ancient world has been attributed to urbanization and dam construction. Cross-border wars and displacement, increasing refugee populations, and poor healthcare services offered in the suburban areas of the Middle East and Central Asia such as Afghanistan, Iran, Iraq, Syria, and Turkey are among the causes of the increase and emergence of this disease (18). The recent drought in the country was also another cause of the increased rate of cutaneous leishmaniasis, which was consistent with our study results.
According to the results of this study on patients referred to leishmaniasis treatment centers affiliated to SBMU in 2010-2019, the highest monthly incidence of the disease was found for November (21.4%) followed by December (17.2%) and October (14.1%). The lowest incidence during the given years was recorded for July (2.7%) followed by April (3.4%). Seasonal variation in the incidence of the disease was clear, so the highest incidence of the disease was 52.7% in autumn, whereas the lowest one was 11.4% in spring. It seems that the most common cause of leishmania in Shahid Beheshti University was due to traveling to endemic parts of the country. In the study by Rassa et al. in Azadegan plain, the highest seasonal prevalence was related to winter (January and February) (11).
Mozaffari and Bakhshizadeh showed that the highest incidence of the disease was recorded in the Yazd-Ardakan plain in September to December, and the highest incidence of the disease was related to autumn (50%), while the lowest incidence of disease was detected in spring (12%) (5).
Many studies have shown the effect of climate and weather on the incidence of leishmaniasis. The Cardenas et al.’s study showed that climatic and ecological changes affected the distribution of leishmaniasis vectors (19). Their study also revealed that the cases of leishmaniasis increased in Northeastern Colombia during El Niño (19). In the study by Dehghani titled Spatial Analysis and Geographic Factors Associated with Cutaneous Leishmaniasis in southern Iran, a high incidence of CL was observed in areas with maximum temperature, mean of temperature, mean of evaporation, sunny days, and wind velocity (20).
According to our study results, the prevalence rate per 100,000 population in 2011 reached 6.97 due to the registration of cases from previous years in the Ministry of Health system, but the increase in the peak rate of leishmaniasis had no effect on this prevalence; the trend developed since then, but this gentle trend in 2017 suddenly increased significantly and then decreased over the past year, which may have been attributable to the decline in travel rate due to the prevalence of COVID-19 in 2019 - 2020 and the following curfew on cities with a high corona prevalence.
In a study by Zeleke et al. at Gondar University Hospital in northwestern Ethiopia, the ten-year data were retrospectively extracted (21). All patients having been referred to the center for cutaneous leishmaniasis over the past ten years were included in the study. According to their findings, no significant difference was detected among data from the previous ten years in terms of the prevalence of cutaneous leishmaniasis (21).
As for the occupation of patients with cutaneous leishmaniasis in the present study, housewives showed the highest rate (21.8%) and ranchers showed the lowest rate (1.3%). As discussed earlier, our study results were consistent with those of other studies in this regard. There was a statistically significant relationship between job and travel history. In the study by Mokhtari and Golmakani in Mashhad, Iran, housewives had the highest incidence (33.5%) (9). In a study by Zhao et al., the highest incidence was recorded for children at home (43%) followed by farmers (27%) and houseworkers (3.9%) (22). The direct and indirect effects of occupation on the incidence of leishmaniasis and exposure to its vectors are undeniable (23). In a systematic and meta-analysis review by Sabzevari et al. titled Cutaneous Leishmaniasis in Iran: A systematic review and meta-analysis, the lowest prevalence was determined for Leishmania tropica with 23% (11 - 38%) while the highest prevalence was found for Leishmania major with 32%. The prevalence of both Leishmania tropica and Leishmania major strains was 60% (48% - 71%), which was not in line with our study result (24).
In the study by Rassa et al. in Azadegan plain, about 59.54% of the patients had a wound and most of their limbs had a hand lesion (28.78%) (11). In the study by Mokhtari and Golmakani in Mashhad, Iran, most of the patients’ limbs had hand lesions with 31.5%, which was greater than that found by our study (9).
5.1. Conclusions
Cutaneous leishmaniasis was still a great health problem in Iran. The prevalence of CL was higher in autumn and among young male travelers to local areas, including Kashan, Isfahan, Semnan and Garmsar, Khuzestan, Khorasan, Qom, and Golestan. Due to the increasing deforestation, continued drought, lack of reduction in the incidences of disease, as well as the presence of indigenous centers in Varamin Plain in southeast of Tehran province, it was recommended that sufficient research attention should be given to the improvement of environment in the marginalized rural areas of Tehran province in order to prevent the local transmission of skin leishmaniasis. It was found necessary to improve the disease surveillance system in high-risk areas in order to provide early diagnosis and optimal treatment. It was also suggested that the people covered by SBMU should improve their knowledge as well as reinforce their attitudes and practices in order to facilitate dealing with this disease and reducing its incidences.
5.2. Limitations
The present study had some limitations. First, the access to weather data for measuring the effects of climate on the occurrence of leishmaniasis was limited. Second, there was not enough variables (e.g., socioeconomic status) in data recording system to evaluate their effects.