1. Background
The course of coronavirus disease 2019 (COVID-19) infection in patients with pre-existing kidney disease, according to the recent literature, is quite severe and associated with high mortality. The patients with chronic kidney disease (CKD) on maintenance hemodialysis have been observed to be highly vulnerable to infections (1), with an increased risk of severe disease and poor outcomes upon infection with COVID-19 (2). According to recent observations, the immunopathology of COVID-19 primarily involves dysregulation of immune response and coagulation abnormalities (3). The older age, the burden of comorbidities, and the frailty of CKD patients on maintenance dialysis, together with inflammatory stress imposed by the infection, result in high vulnerability and worsen the outcomes (4, 5). The clinical characteristics and in-hospital course of CKD patients with COVID-19 infection and outcomes in terms of in-hospital mortality have been studied retrospectively based on the medical records. Comparing survivors with non-survivors in terms of clinical and laboratory parameters has led to identification of several factors which may affect and predict the clinical course of COVID-19 infection in these patients.
2. Objectives
This study attempted to highlight these factors in the Indian patients with CKD on maintenance hemodialysis who had been admitted to a COVID-19 hospital in India during both waves of the COVID-19 pandemic from July 2020 to June 2021.
3. Methods
This retrospective cohort study was conducted at the National Cancer Institute (NCI), All India Institute of Medical Sciences (AIIMS) in Jhajjar, Haryana, India. The institute had been designated as a COVID hospital during the pandemic. The study protocol was approved by the Institute Ethics Committee (IEC) of AIIMS, New Delhi. The design and draft of the protocol were formulated in accordance with the checklist of Strengthening the Reporting of Observational Studies in Epidemiology (STROBE), and they were approved by the IEC under the ethical approval code of IEC/152/3/2021 (link: 14.139.245.45:8082/iecaiims/Pages/frmViewDocuments.aspx?TId=IEC/152/3/2021). All consecutive patients who were documented cases of CKD on maintenance hemodialysis and had COVID-19 infection confirmed by real-time polymerase chain reaction (RT-PCR) test from the nasopharyngeal swab sample were included in the study. All patients with inadequate data were excluded from the study.
The retrospective clinical data were extracted from the case sheets, screening documents, and treatment records. The laboratory results were abstracted into excel datasheet from the hospital’s electronic patient information portal. The demographic details, comorbidities, and various other parameters included oxygen saturation at presentation, hemodialysis sessions, intra or post-dialysis complications, administered medical treatment, course of the disease during hospitalization, baseline laboratory, length of hospital stay, and outcomes in terms of discharge from the hospital or death during hospitalization.
3.1. Coronavirus Disease 2019 Severity Definitions Based on Institutional Protocol
3.1.1. Mild Coronavirus Disease 2019
Patients with oxygen saturation (SPO2 on pulse-oximetry) ≥ 94% on room air with or without breathlessness or other symptoms suggestive of COVID-19 such as fever, sore throat, myalgia, fatigue etc.
3.1.2. Moderate Coronavirus Disease 2019
Patients having symptoms of infection and breathlessness with the respiratory rate (RR) ≥24/min and SPO2 on pulse-oximetry ≤ 94% on room air.
3.1.3. Severe Coronavirus Disease 2019
Patients having symptoms of infection and breathlessness with the RR ≥ 30 with SPO2 on pulse-oximetry ≤ 90% on room air.
3.2. Statistical Analysis
The collected data were cross-checked, verified, and imported to MS Excel software version 16.0 (Microsoft Inc.). SPSS Version 24 (SPSS Inc, Chicago IL, USA) was used for statistical analysis. Median (interquartile, interquartile range (IQR) 25th - 75th) was used to present quantitative clinical data, and categorical data were presented as numbers and proportions. The data summary was tabulated for comparison, and statistical significance was checked using Fisher’s exact tests and Mann-Whitney U test for qualitative and quantitative data, respectively. A P-value less than 0.05 was considered significant.
4. Result
4.1. Baseline Clinicodemographic Characteristics of Patients in Both Waves of Coronavirus Disease 2019
During the first wave of COVID-19, a total of 43 patients underwent dialysis at our facility, 36 of them were CKD on maintenance hemodialysis; and 7 of them were acute kidney injury (AKI) patients who were excluded from the study. One patient was also excluded from the study because of inadequate data. Out of these 35 patients, 8 patients (22.9%) died during the hospital stay, and 27 patients (77.1%) were discharged. In the second wave, a total of 20 patients underwent hemodialysis, of whom only 5 patients were CKD on maintenance dialysis while remaining 15 patients were AKI patients. Three of them (60%) were discharged, and 2 of them (40%) didn’t survive. All patients with in-hospital mortality from both waves died from the complications of COVID-19 infection.
The baseline clinico-demographic characteristics of the patients from the first wave are shown in Table 1. The median (IQR) age of the patients was 51 (IQR, 42.5 - 63.5) years, ranging from 28 to 84 years with 25.7% of patients over 65 years of age. The median (IQR) age in the second wave was 62 (IQR, 53 - 75) years, ranging from 50 to 77 years. Hypertension was most common among co-morbidities, with a prevalence of 91.4%, followed by diabetes, with a prevalence of 34.3%. The median (IQR) days between symptom onset to hospitalization was 5 (IQR, 2 - 7.5) days. The most common presenting symptoms were shortness of breath (SOB) (62.9%), fever (54.3%), and cough (45.7%) in descending order. The severity of infection was mild in most cases (42.9%), but moderate (28.6%) and severe (28.6%) in the rest. Twenty-three patients (65.7%) required oxygen supplementation at admission to the hospital. A similar clinico-demographic profile was observed in 5 patients of the second wave of COVID-19.
| Variables | Total (n = 35) | Survivors (n = 27) | Mortality (n = 8) | P-Value |
|---|---|---|---|---|
| Age (y) | 51 (42.5, 63.5) | 49 (42, 55) | 70 (54, 74) | 0.016 b |
| Gender | 0.139 | |||
| Male | 23 (65.7) | 16 (59.3) | 7 (87.5) | |
| Female | 12 (34.2) | 11 (40.7) | 1 (12.5) | |
| Primary kidney disease | 0.676 | |||
| HTN-NP | 17 (48.6) | 13 (48.1) | 4 (50.0) | |
| Diabetic-NP | 10 (28.6) | 7 (25.9) | 3 (37.5) | |
| PN | 8 (22.9) | 7 (25.9) | 1 (12.5) | |
| Comorbidity | 0.778 | |||
| Hypertensive | 32 (91.4) | 25 (92.6) | 7 (87.5) | |
| Diabetic | 12 (34.3) | 8 (29.6) | 4 (50.0) | |
| CAD | 5 (14.3) | 3 (11.1) | 2 (25.0) | |
| Others | 11 (31.4) | 8 (29.6) | 3 (37.5) | |
| Presenting symptoms | 0.744 | |||
| Shortness of breath | 22 (62.9) | 16 (59.3) | 6 (75.0) | |
| Fever | 19 (54.3) | 13 (48.1) | 6 (75.0) | |
| Cough | 16 (45.7) | 11 (40.7) | 5 (62.5) | |
| Others | 13 (37.1) | 11 (40.7) | 2 (25.0) | |
| Duration of symptoms before test positivity (days) | 5 (2,7.5) | 5 (2,10) | 3.5 (2,5) | 0.183 |
| Severity of infection at admission | 0.004 b | |||
| Mild | 15 (42.9) | 14 (51.9) | 1 (12.5) | |
| Moderate | 10 (28.6) | 9 (33.3) | 1 (12.5) | |
| Severe | 10 (28.6) | 4 (14.8) | 6 (75.0) | |
| Chest radiograph | 0.005 b | |||
| Bi-lateral infiltrates | 20 (57.1) | 12 (44.4) | 8 (100) | |
| SPO2: Room air at admission | 97 (95,98) | 97 (95,98) | 97.5 (80,99.5) | 0.649 |
| Oxygen support at admission | 0.020 b | |||
| Present | 23 (65.7) | 15 (55.6) | 8 (100) | |
| Absent | 12 (34.3) | 12 (44.4) | 0 (0) | |
| Pre-COVID dialysis duration (y) | 0.75 (0.5,4) | 0.5 (0.25,1) | 1 (0.5,4.2) | 0.081 |
Abbreviations: IQR, interquartile range, HTN-NP, hypertensive nephropathy; PN, primary nephropathy; CAD, coronary artery disease; COVID, coronavirus disease.
a Values are expressed as median (interquartile range) or No. (%).
b Statistical significance i.e., P-value less than 0.05
4.2. Baseline Laboratory Parameters
The baseline findings in hematologic parameters were a decreased mean absolute lymphocyte counts (ALC), a high neutrophil-lymphocyte ratio (NLR), and a mild decrease in platelet counts. A baseline high blood urea and serum creatinine values were observed. The markers of inflammation, interleukin-6 (IL-6), C-reactive protein (CRP), procalcitonin (PCT), fibrinogen, ferritin, and D-dimer were also high at admission. The coagulation profile showed near-normal PT, APPT, and INR. A similar trend was observed in patients from the second wave with slightly higher baseline total leukocyte count (TLC), NLR, blood urea, and serum creatinine levels. The baseline chest X-ray (CXR) was suggestive of bilateral infiltrates in 57% and 80% of the patients in the first and second wave, respectively. The median (IQR) values of baseline laboratory findings in survivors and non-survivor patients in the first wave are shown in Table 2.
| Variables (Normal Range) | Outcome | P-Value | |
|---|---|---|---|
| Survivors (n = 27) | Mortality (n = 8) | ||
| Hb (13 - 17 g/dL) | 8.5 (7.5, 10.5) | 8.9 (7.7, 10.3) | 0.369 |
| TLC (4 - 10 × 103/μL) | 7.5 (5.2, 12.6) | 11.4 (9.8, 21.1) | 0.048 b |
| Lymphocyte (11 - 30 × 102/μL) | 11.1 (7, 15.8) | 4.2 (3, 7.6) | 0.011 b |
| NLR (1 - 3) | 7.1 (5.7, 12.1) | 21.8 (11.8, 29.8) | 0.045 b |
| Platelet (150 - 400 × 103/μL) | 137 (113, 276) | 135 (112, 236) | 0.844 |
| PT (10.2 - 13.2 (s)) | 12.8 (11.6, 14.2) | 12 (10.8, 13.5) | 0.146 |
| APTT (25.4 - 38.4 (s)) | 32.8 (28.3, 35.3) | 30.5 (28.5, 35.3) | 0.937 |
| INR (< 1.1) | 1.1 (1.0, 1.2) | 1.0 (0.9, 1.1) | 0.157 |
| Fibrinogen (180 - 350 mg/dL) | 339 (276, 449) | 392 (332, 499) | 0.013 b |
| D-dimer (< 500 ng/mL) | 330 (110.8, 808.5) | 1485 (529, 2856) | 0.146 |
| Troponin (< 0.04 ng/mL) | 0.04 (0.01, 0.09) | 0.11 (0.08, 1.92) | 0.530 |
| CPK (33 - 211 IU/L) | 161 (57.2, 271) | 169.5 (65.5, 1125.7) | 0.844 |
| Ferritin (22 - 322 ng/mL) | 1204 (340, 1650) | 1150 (444, 2383) | 0.252 |
| CRP (0 - 0.5 mg/dL) | 9.1 (3.2, 12.2) | 10.5 (3, 19.2) | 0.346 |
| IL-6 (0 - 4.4 pg/mL) | 22.5 (5.7, 104.6) | 66.6 (10.7, 184.6) | 0.028 b |
| Procalcitonin (< 0.1 ng/mL) | 0.6 (0.2, 4.4) | 0.9 (0.2, 9) | 0.037 b |
| LDH (120 - 246 U/L) | 349.5 (254, 520.2) | 453 (224, 612) | 0.969 |
| Urea (< 50 mg/dL) | 70.6 (27.8, 119.8) | 160.1 (141.7, 355.2) | 0.002 b |
| Creatinine (0.7 - 1.3 mg/dL) | 8.5 (4.9, 9.8) | 5.1 (4.1, 8.5) | 0.246 |
| Bilirubin (0.3 - 1.2 mg/dL) | 0.26 (0.21, 0.53) | 0.24 (0.19, 0.28) | 0.270 |
| SGPT (10 - 49 U/L) | 21 (9.7, 35.5) | 24.3 (16.8, 34.7) | 0.569 |
| SGOT (< 34 U/L) | 23 (15.6, 48) | 80.1 (59.7, 104.7) | 0.001 b |
| Albumin (3.2 - 4.8 g/dL) | 3.4 (3.1, 3.8) | 3 (2.7, 3.2) | 0.030 b |
| Sodium (132 - 146 mmol/L) | 137 (133, 139) | 135 (133, 141) | 0.844 |
| Potassium (3.5 - 5.5 mmol/L) | 4.1 (3.7, 5.1) | 5.1 (4.3, 6.1) | 0.041 b |
| Calcium (8.7 - 10.4 mg/dL) | 7.4 (6.7, 8.8) | 7.6 (7.3, 7.9) | 0.906 |
| Phosphorus (2.4 - 5.1 mg/dL) | 5.7 (3.9, 6.7) | 3.9 (3, 8.1) | 0.582 |
Abbreviations: TLC, Total leukocyte count; NLR, neutrophil-lymphocyte ratio; CRP, C-reactive protein; IL-6, interleukin-6; SGOT, serum glutamic oxaloacetic transaminase.
a Values are expressed as median (interquartile range).
b Statistical significance i.e., P-value less than 0.05
4.3. In-hospital Course and Outcomes
The severity of the disease increased in a few patients after an initial admission, which necessitated the transference of the patients from the ward or high dependency unit (HDU) to ICU, escalation of medical treatment, upgrade of oxygen therapy, and non-invasive or invasive mechanical ventilatory support. A total of 6 patients (17.1%) required invasive mechanical ventilation (IMV) with a median (IQR) duration of 4 (IQR, 0.2 - 4.7) days on the ventilator. In the second wave, 2 out of 5 patients required IMV. Those patients requiring IMV during hospitalization couldn’t survive. All patients underwent hemodialysis, and only one patient (2.9%) had post-dialysis hypotension and arrhythmia managed with appropriate pharmacological measures. In the second wave, three patients (60%) had hypotension requiring albumin and vasopressors to maintain mean blood pressure. Eight patients (22.9%) out of a total of 35 ones in the first wave as well as 2 out of 5 patients (40%) had in-hospital mortality. The cause for mortality was found to be the complication of the severe COVID disease leading to acute respiratory distress syndrome (ARDS) with multiorgan dysfunction syndrome (MODS) with or without refractory shock. The in-hospital management and outcomes are shown in Table 3.
| Variables | Total (n = 35) | Survivors (n = 27) | Mortality (n = 8) | P-Value |
|---|---|---|---|---|
| Initial bed allocation | ||||
| HDU | 17 (48.6) | 16 (59.3) | 1 (12.5) | < 0.001 b |
| ICU | 6 (17.1) | 0 (0) | 6 (75.0) | |
| Ward | 12 (34.3) | 11 (40.7) | 1 (12.5) | |
| Oxygen therapy during hospital stay | ||||
| FM/NRBM | 5 (14.2) | 5 (100) | 0 (0) | < 0.001 b |
| HFNC | 9 (25.7) | 9 (100) | 0 (0) | |
| NIV | 6 (17.1) | 4 66.60 | 2 (33.3) | |
| IMV | 6 (17.1) | 0 (0) | 6 (100) | |
| None | 9 (25.7) | 9 (100) | 0 (0) | |
| Steroid use | ||||
| Dexamethasone | 19 (54.3) | 17 (63.0) | 2 (100) | < 0.001 b |
| Methylprednisolone | 7 (20) | 1 (3.7) | 6 (75.0) | |
| None | 9 (25.7) | 9 (33.3) | 0 (0) | |
| Treatment | ||||
| Antibiotics | 31 (88.6) | 23 (85.2) | 8 (100) | 0.247 |
| Antiviral | 22 (62.9) | 14 (51.9) | 8 (100) | 0.013 b |
| Antifungal | 2 (5.7) | 0 (0) | 2 (25.0) | 0.007 b |
| Supportive | 35 (100) | 27 (100) | 8 (100) | - |
| Haemodialysis | 35 (100) | 27 | (100) | 8 (100) |
| Haemodialysis sessions (No.) | 3 (2,5) | 3 (2,5) | 3(1,4) | - |
| Duration ward/HDU | 11 (5,14) | 11 (8,15) | 0 (0, 11.7) | 0.001 b |
| Duration: ICU | 3.1 (0,4) | 0 (0,0.5) | 7 (4,14) | 0.003 b |
| Duration: Mechanical ventilation | 0.8 (0, 2.5) | 0 (0,0) | 4 (0.2,4.7) | 0.001 b |
| Hospital stay | 13 (10.5, 17.5) | 13 (11,17) | 14.5 (4.2,18.7) | 0.953 |
| Invasive mechanical ventilation | ||||
| Present | 6 (17.1) | 0 (0) | 6 (75.0) | < 0.001 b |
| Absent | 29 (82.9) | 27 (100) | 2 (25.0) | |
| Off-label/EUA therapy | ||||
| Present | 21 (60) | 14 (51.9) | 7 (87.5) | 0.071 |
| Absent | 14 (40) | 13 (48.1) | 1 (12.5) | |
| Post dialysis complications | ||||
| Present | 1 (2.9) | 0 (0) | 1 (12.5) | 0.062 |
| Absent | 34 (97.1) | 27 (100) | 7 (87.5) |
Abbreviations: HDU, High dependency unit, ICU, intensive care unit, FM, face mask, NRBM, non-rebreathing mask, HFNC, high flow nasal cannula, NIV, non-invasive ventilation, IMV, invasive mechanical ventilation, EUA, emergency use authorization.
a Values are expressed as median (interquartile range) or No. (%).
b Statistical significance i.e., P-value less than 0.05
4.4. A Comparison of Clinicodemographic Characteristics and Outcomes Between Survivors and Non-survivors
The comparison of survivors and non-survivors in terms of clinicodemographic features and in-hospital management and outcomes is presented in Tables 1 and 3, respectively. There was a significant difference between non-survivors and survivors regarding median (IQR) age (70 [IQR, 54 - 74] years and 49 [IQR, 42 - 55] years, P = 0.016, respectively). The severity of COVID-19 infection at admission was higher in non-survivor group. Furthermore, 75% of the patients had severe COVID-19 disease at admission in the non-survivor group with the presence of bilateral infiltrates on CXR and requirement of oxygen and respiratory support for all patients at admission. In the survivor group, 52% of the patients had mild disease at admission, 44% of whom were not in need of oxygen or respiratory support. All the patients in the non-survivors group received higher antibiotics, antivirals, antifungals, steroids, and off-label and emergency use authorization (EUA) drugs following the escalation of the medical treatment. All 6 patients who required IMV during the first wave and 2 patients in the second wave failed to survive. There were no differences between the two groups regarding median (IQR) days of the hospital stay length (13 [IQR, 11 - 17] versus 14.5 [IQR, 4.2 - 18.7] days, P = 0.95).
4.5. A comparison of Baseline Laboratory Parameters Between Survivors and Non-survivors
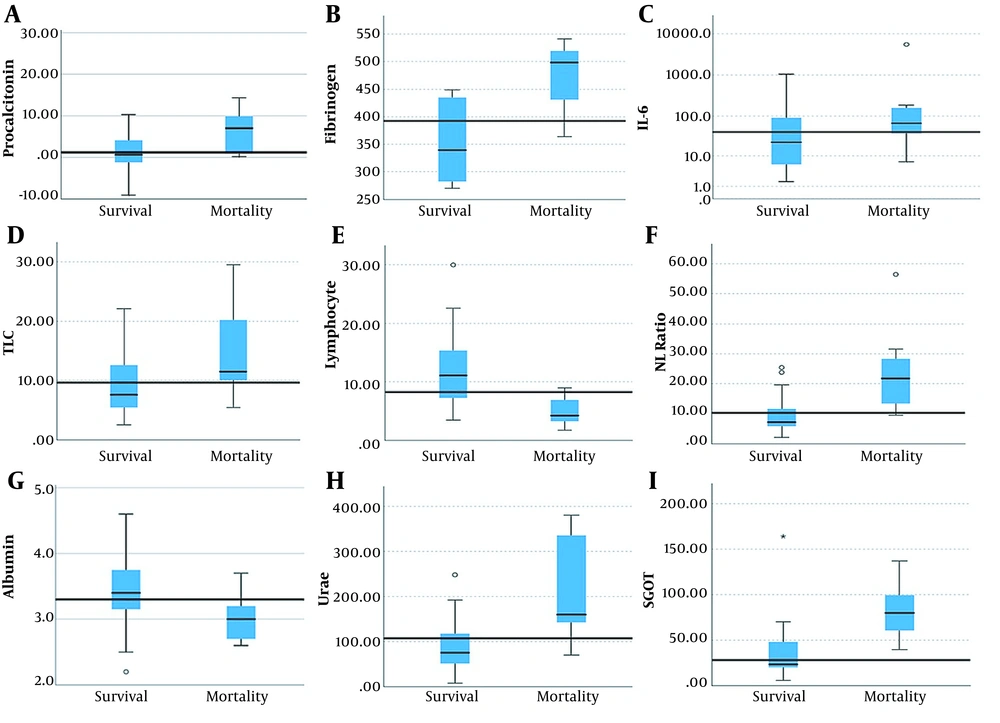
The comparison of baseline laboratory parameters is shown in Table 2 and Figure 1. There was a significant difference in baseline PCT and IL-6 levels. In survivors vs. non-survivors, the median (IQR) values of PCT were 0.6 (IQR, 0.2 - 4.4) ng/mL vs. 0.9 (IQR, 0.2 - 9) ng/mL (P =0.03), while the IL-6 values were 23 (IQR, 5.7 - 105) vs. 67 (IQR, 11 - 185) pg/mL (P = 0.03). The values of other markers of inflammation like CRP and D-dimers were also higher in non-survivors, but the difference was not significant. Blood urea was significantly raised in non-survivors with a median (IQR) value of 160 (IQR, 142 - 355) mg/dL) compared to survivors 71 (IQR, 28 - 120) mg/dL (P = 0.002). Baseline fibrinogen and serum glutamic oxaloacetic transaminase (SGOT) levels were also significantly higher, while serum albumin was significantly low in non-survivors compared to survivors (3 [IQR, 2.7 - 3.2] versus 3.4 [IQR, 3.1 - 3.8] g/dL, P = 0.03). A significantly higher TLC and NLR as well as a lower absolute lymphocyte count were observed in non-survivors.
The comparison of survivors and non-survivors regarding median laboratory parameters. A, Procalcitonin (PCT); B, Fibrinogen; C, Interleukin-6 (IL-6); D, Total leukocyte count (TLC); E, Absolute lymphocyte count (ALC); F, Neutrophil lymphocyte ratio (NLR); G, Albumin; H, Blood urea; I, Serum glutamic oxaloacetic transaminase (SGOT)
5. Discussion
This retrospective study aimed at determining the various clinical and biochemical predictors of in-hospital mortality in this group of preexisting CKD patients on maintenance hemodialysis infected with the COVID-19 virus. All patients underwent hemodialysis during the hospital stay; as for the modality, a majority of the patients received intermittent hemodialysis (IHD) treatment, and only a few of them underwent sustained low-efficiency daily dialysis (SLEDD). None of the patients from both waves received continuous renal replacement therapy (CRRT). The data obtained from the 35 patients from the first wave of COVID-19 were analyzed because there were only 5 CKD patients from the second wave. A recent retrospective analysis from Indian ICUs during both waves of the pandemic also observed a similar trend, with a significantly lesser number of patients with pre-existing renal dysfunction during the second wave with a corresponding lesser need for hemodialysis (6). The number of patients undergoing dialysis for AKI (non-CKD) were higher in the second wave compared with that in the first wave (68.4 % [13 out of 19 patients] versus 18.6% [8 out of 43 patients], respectively). The obtained data revealed that most patients were middle-aged, with a median (IQR) age of 51 (IQR, 43 - 64) years, and with one or more co-morbidities plus CKD that had predisposed them to a higher risk of severe viral infection. More than 50% of the patients (57.2%) presented with moderate to severe disease whose most reported symptoms were shortness of breath (62.9%), fever (54.3%), and cough (45.7%) requiring a treatment with oxygen support via a variety of oxygen delivery devices at admission. Thus, the symptoms of presentation in this patient cohort were not different than the symptomatic presentation in the general population; there was also no significant difference between survivors and non-survivors in terms of symptomatology. A high baseline level of inflammatory markers like CRP, IL6, PCT, ferritin, and LDH was observed in majority of the patients. Moreover, 75% of the patients presenting with severe disease at admission died, 6 of whom required invasive mechanical ventilation (IMV) during ICU stay. A significantly elevated TLC, NLR, IL-6, PCT, serum fibrinogen, blood urea, SGOT, as well as a significantly low absolute lymphocyte count, and albumin level were found in patients who had experienced unfavorable outcomes. These findings suggested that a low albumin level, coagulation abnormalities, severe inflammation, and deteriorating renal function increased the risk of mortality in these patients. A similar trend of the raised inflammatory markers and high blood urea levels were observed in patients with unfavorable outcomes in the second wave, although the data were not analyzed further due to the very small sample size (5 patients only). A mortality of 23% was observed in our patients, which corresponded to the similar mortality range of 16 - 32% reported for a similar group of patients in several recent studies (2-5). The complication of severe COVID-19 disease leading to ARDS with or without multiorgan dysfunction (MOD) was the cause of mortality in all the patients, which was consistent with the observation reported by other previous studies (7-9).
The patients with CKD on maintenance dialysis have an impaired immune function (10). The elevation of the inflammatory markers’ levels has suggested that they induce an immunological response against the coronavirus infection, and the cytokines play a key role in its immunopathology (11). A high CRP level is associated with worse outcomes (2-5, 7, 12), and a similar finding in our study supported the evidence although there was no significant elevation in non-survivors. The elevations in IL-6 and PCT were also associated with in-hospital death in infected patients (3, 13, 14). The role of IL-6 and CRP as predictors of all-cause mortality in dialysis patients had already been highlighted in previous studies (3, 15-17). Thus CRP, IL-6, and procalcitonin levels may have helped to predict the progression of the infection severity in CKD patients on maintenance dialysis.
Regarding coagulation abnormalities, elevated fibrinogen, D-dimer was observed in a majority of our patients, with a significant elevation of fibrinogen in non-survivors. The presence of coagulation abnormalities has been well established by previous studies on patients with COVID-19 (3, 9, 18). Thus, coagulation abnormalities had a higher incident rate in CKD patients on maintenance dialysis, and a significant derangement was associated with poor outcomes. Other laboratory parameters like increased TLC, NLR, and lymphocytopenia are also associated with poor outcomes (3-5, 7). Similar findings were also observed in our study, indicating that TLC, and NLR was significantly elevated in the in-hospital mortality group. According to the results from routine investigations, baseline high blood urea, SGOT, and potassium levels were associated with higher mortality in this group of patients (2, 5), which were in agreement with our findings. A low albumin level was also associated with poor outcomes in these patients (2-5), which was also consistent with one of our significant findings.
Our study faced some limitations. First, a small sample size was used in our study, and, therefore, the findings of our study may not be generalizable to the whole population of CKD patients on maintenance dialysis. Although our observations were consistent with the findings of similar studies conducted elsewhere in the world, a few prognostic factors were unique in this group of patients. Second, the retrospective data retrieval may have been affected by the bias inherent to data selection. Third, the number of CKD patients undergoing dialysis in the second wave of COVID-19 was very small. Therefore, the clinical characteristics and outcomes of the first and second waves were not compared, and no definite conclusion was drawn about this aspect. This particular group of patients has been studied in the literature mainly retrospectively; therefore, similar studies with a prospective design with an appropriate sample size should be conducted in the future to provide a more reasonable interpretation and to prove that the observed prognostic factors are valid.
5.1. Conclusions
Our findings and the available literature to date lead us to conclude that CKD patients on maintenance hemodialysis are more susceptible to severe disease following infection with Coronavirus due to the presence of multiple co-morbidities, frailty, and aging. In-hospital mortality or poor outcomes are associated with several clinical factors such as older age, severe disease at presentation, need of oxygen and respiratory support, and baseline biochemical parameters such as elevated inflammatory markers, IL-6, PCT, fibrinogen level, and low albumin level. These clinical & biochemical parameters can serve as predictors of in-hospital mortality or poor outcomes in this group of patients infected with COVID-19.