1. Background
Antibiotic resistance in bacteria is a major problem encountered in routine care of hospitalized patients. Initial predominance of gram-positive organisms in hospital acquired infections (HAIs) is now surfaced by isolation of gram-negative organisms, which demanded use of broad-spectrum antimicrobials as a therapeutic option. This led to increased usage of advanced generation cephalosporins and carbapenems in treatment of gram-negative hospital acquired pathogens, further allowing these pathogens to explore the resistance mechanisms against the newer antimicrobial agents. Since year 2000, a group of multidrug resistant organisms (MDROs) collectively known as “ESKAPE” (E. faecium, S. aureus, K. pneumoniae, A. baumannii, P. aeruginosa and Enterobacter species) bugs are dominating the hospital environments (1).
Ventilator-associated Pneumonia (VAP) is defined as pneumonia that occurs 48 - 72 hours or thereafter following mechanical ventilation, characterized by the presence of a new or progressive infiltrate on chest X-ray, signs of systemic infection (fever and altered white blood cell count), changes in sputum characteristics, and detection of a causative microorganism (2). It is the second most common nosocomial infection at the intensive care unit (ICU) and is estimated to occur in 9% to 27% of all mechanically ventilated patients, with the highest risk being early in the course of hospitalization (3). Among neonates it accounts for 6.8% to 32.2% of health care-acquired infections and is associated with prolonged hospitalization, increased health care costs, and high attributable mortality (4). Various factors like aspiration of secretions, colonization of the aero digestive tract, the use of contaminated equipment, improper aseptic techniques, or medications predispose development of VAP apart from various other risk factors (5).
Etiology of VAP has been reported as being polymicrobial. Both gram-positive and gram-negative organisms are responsible for VAP. A number of studies from India have reported Pseudomonas spp., Acinetobacter spp., Escherichia coli, Klebsiella pneumoniae and Staphylococcus aureus as common VAP pathogens, with varying prevalence. Many of these pathogens especially, Pseudomonas spp., Acinetobacter spp. and even Enterobacteriaceae are often multidrug-resistant (6). The type of organism that causes VAP usually depends upon duration of mechanical ventilation. In general, early VAP is caused by pathogens that are sensitive to antibiotics while, late onset VAP is caused by multidrug resistant organisms (MDRO) making the treatment options more complex (3). Also, the agents of VAP vary with different patient populations and types of ICUs. Therefore, the local microbial flora causing VAP and their resistance profile needs to be studied in each setting to guide more effective and rational utilization of antimicrobial agents.
2. Objectives
The present study was planned to describe the trend in the isolation of multidrug resistant pathogens causing VAP in a newly constructed neonatal intensive care unit (NICU).
3. Methods
This study represents data obtained retrospectively over a period of six months from the establishment of a newly constructed NICU at a Tertiary care hospital, from January to July 2015.
The newborns (admitted to the NICU during the study period), which were clinically diagnosed with ‘suspected’ ventilator associated pneumonia, were included. Samples of endotracheal aspirates collected from these infants for bacteriological confirmation of VAP were analyzed. All the samples were gram stained and cultured by semi-quantitative method on 5% Sheep Blood agar and MacConkey’s Medium. Further identification and antibiotic susceptibility testing of the isolates was done as per standard protocols. Repeated sampling at an average interval of two to three days was done for some of the newborns. The data obtained on type of microbial flora isolated with their antibiotic susceptibility profile was compiled and analyzed.
4. Results
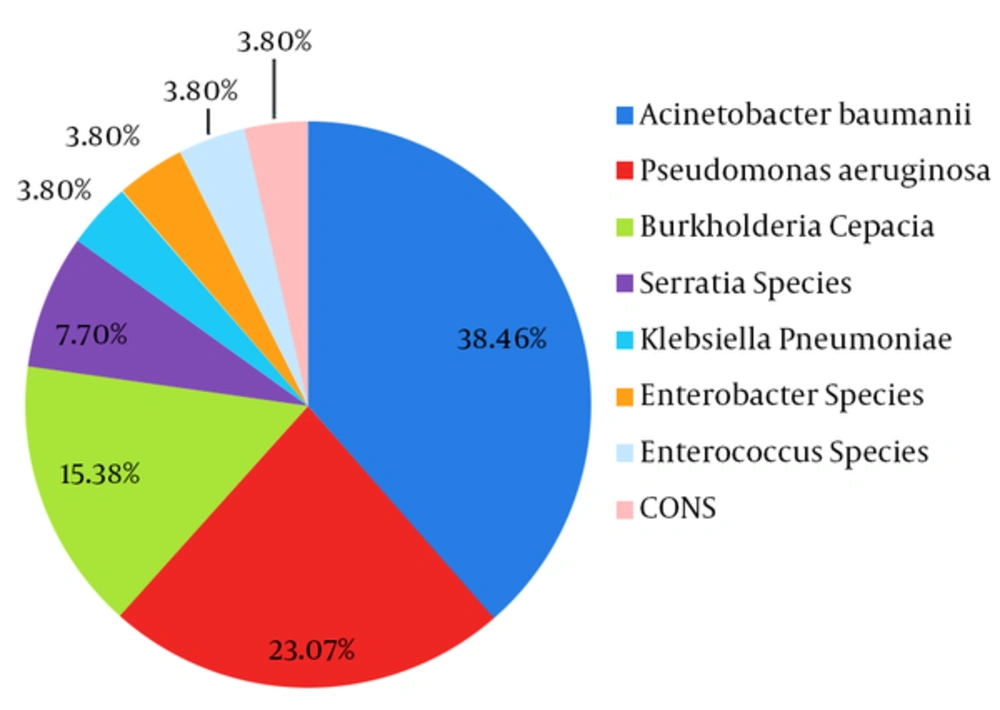
A total of seven newborns were put on mechanical ventilation during the study period. Out of seven, two infants had no microbiological evidence of VAP. Among the remaining five cases, a total of 26 VAP pathogens were isolated on separate occasions. Acinetobacter baumannii was the most frequently isolated organism in 38.46% of samples (10 out of 26), followed by Pseudomonas aeruginosa in 23.07% of samples (6 out 26) (Figure 1). Samples from four out of five newborns (57.14%), showed predominant growth of Acinetobacter baumannii and Pseudomonas aeruginosa on either single or multiple occasions. Both these strains were resistant to almost all first and second line antibiotics and were sensitive only to polymyxin B and colistin (Table 1). In two patients, both organisms were isolated together in samples collected at initial stages. In another patient, combined growth of Klebsiella pneumoniae (Extended Spectrum Beta-Lactamase (ESBL) producer, susceptible to imipenem) and Enterococcus faecalis (susceptible to vancomycin) was obtained only on one occasion. Other gram-negative organisms that were isolated with less frequency were Burkholderia cepacia, Serratia species, Klebsiella pneumoniae, and Enterobacter species, which also carried resistance to multiple antibiotics (Table 1). None of the gram-negative bacteria except Burkholderia cepacia and Serratia species demonstrated colistin and polymyxin B resistance. Gram-positive cocci like Coagulase negative Staphylococci (CONS) and Enterococci were equally multidrug resistant and were susceptible only to vancomycin and linezolid.
| Patient No. | Total Samples Collected | Sample Number | Organism Isolated | Sensitivity Pattern | |
|---|---|---|---|---|---|
| Resistant | Sensitive | ||||
| 1 | 01 | 01 | Acinetobacter baumannii | A, A/S, G, Cip, Ctx, Cpm, Ak, Caz, Cot, Az, Imp, Mero | PB, CL |
| Pseudomonas aeruginosa | G, Cip, Ctx, Cpm, Ak, Caz, Cot, Az, Imp, Mero | PB, CL | |||
| 2 | 08 | 1-3 | Acinetobacter baumannii | A, A/S, G, Cip, Ctx, Cpm, Ak, Caz, Cot, Az, Imp, Mero | PB, CL |
| Pseudomonas aeruginosa | G, Cip, Ctx, Cpm, Ak, Caz, Cot, Az, Imp, Mero | PB, CL | |||
| 4 and 5 | Pseudomonas aeruginosa | G, Cip, Ctx, Cpm, Ak, Caz, Cot, Az, Imp, Mero | PB, CL | ||
| Serratia species | PB, CL | G, Cip, Ctx, Cpm, Ak | |||
| 6 - 8 | Burkholderia cepacia | PB, CL, Ca, Cpm, Az | Ipm, Pip+Tz, Cot, Gen, Cip | ||
| 3 | 03 | 1 and 2 | Acinetobacter baumannii | A, A/S, G, Cip, Ctx, Cpm, Ak, Caz, Cot, Az, Imp, Mero | PB, CL |
| 3 | Acinetobacter baumannii | A, A/S, G, Cip, Ctx, Cpm, Ak, Caz, Cot, Az, Imp, Mero | PB, CL | ||
| Burkholderia cepacia | PB, CL, Ca, Cpm, Az | Ipm, Pip+Tz, Cot, G, Cip | |||
| 4 | 01 | 01 | Klebsiella pneumoniae | A,G, Cip, Cxm, Cpm, Ctx, CaZ, Cot, Pip | Imp, Pip+Tz |
| Enterococcus faecalis | Pen, Tetr, cot, cip, ctx, A | Van, Lz | |||
| 5 | 03 | 01 | Acinetobacter baumannii | A, A/S, G, Cip, Ctx, Cpm, Ak, Caz, Cot, Az, Imp, Mero | PB, CL |
| 02 | Acinetobacter baumannii | A, A/S, G, Cip, Ctx, Cpm, Ak, Caz, Cot, Az, Imp, Mero | PB, CL | ||
| CONS | Pen, E, Clinda, Cot, Oxa, Cip | Van, Lz | |||
| 03 | Acinetobacter baumannii | A, A/S, G, Cip, Ctx, Cpm, Ak, Caz, Cot, Az, Imp, Mero | PB, CL | ||
| Enterobacter aerogenes | A, G, Cip, Ctx, Cpm, Ak, Caz, Cot, Az, Imp, Mero | PB, CL | |||
Abbreviations: A, Ampicillin; A/S, Ampicillin-Sulbactam; Ak, Amikacin; AZ, Aztreonam; Caz, Ceftazidime; Cip, Ciprofloxacin; CL, Colistin; Clinda, Clindamycin; Ctx, Cefotaxime; Cot, Cotrimoxazole; Cpm, Cefepime; E, Erythromycin; G, Gentamicin; Imp, Imipenem; Lz, Linezolid; Mero, Meropenem; Oxa, Oxacillin; PB, Polymyxin B; Pen, Penicillin; Pip+Tz, Piperacillin-Tazobactam; Van, Vancomycin.
In the newborns that were initially infected with A. baumannii and P. aeruginosa, a sudden shift in the isolation pattern of organisms was observed. Established pathogens like MDR (sensitive to polymyxins) A. baumannii and P. aeruginosa were drastically replaced by intrinsically polymyxin resistant organisms, like Serratia species and Burkholderia cepacia.
5. Discussion
Ventilator-associated Pneumonia is the second most common hospital-acquired infection among pediatric and NICU patients occurring in 3% to 10% of ventilated pediatric ICU (PICU) patients overall (7). Early onset VAP occurring within four days of intubation is usually attributable to antibiotic sensitive pathogens, while late onset VAP is more likely caused by MDR bacteria that emerge after four days of intubation (3).
Consistent with many other studies (6, 8, 9), this report also highlights the presence of gram negative bacteria especially, gram negative nil fermenters like Acinetobacter and Pseudomonas as more usual causes of VAP as compared to gram positive organisms, which used to be the major culprits in previous decades. Moreover, all the gram-negative organisms that were isolated in the present study were resistant to multiple drugs sparing only the reserved antibiotics like carbapenems and polymyxins. While, all gram-positive organisms showed vancomycin and linezolid, as the only viable alternative to treat these infections.
Colonization and infection by MDRO is driven by forces centering the hospital environment like, selection pressure offered by high dosage and long term usage of antibiotics, immunocompromised state in hospitalized patients and cross infections from a variety of human and nonhuman sources in the hospital (10). The patients with bacteriologically confirmed VAP in the present study had been on empiric antibiotic therapy for various other co morbidities prior to development of VAP. This clearly reflects the role of long-term use of broad spectrum antibiotics, as a major risk factor for development of late onset VAP by MDRO, especially in ICU settings. Other risk factors for VAP include prematurity, very low birth weight, severe underlying disease, prolonged duration of mechanical ventilation, use of wide spectrum antibiotics, prolonged hospital stay, inadequate pulmonary toilet, and extensive use of invasive devices and procedures.
An interesting fact that was noticeable from these observations was that, after long term presence of polymyxin-sensitive Acinetobacter and Pseudomonas in repeated samples from two newborns, the flora suddenly and drastically changed to organisms of less pathogenic potential like Serratia species and Burkholderia cepacia. Both these organisms are known for their intrinsic resistance exhibited towards polymyxins. It is quite possible that antibiotic selection pressure exerted by long term use of polymyxins in these patients might have selected them during therapy. This has given them the opportunity to establish themselves as more troublesome pathogens further complicating the treatment decisions.
Hospital environments are potential reservoirs for dangerous pathogens, which have the tendency to acquire multidrug resistant genes in the due course of time. However, the possibility of circulating antibiotic resistant clones in a relatively new establishment is rare. Observations from the current study in the form of isolation of MDR pathogens from five out of seven patients within a span of mere six months, after a new establishment of NICU, were a cause of concern. To unveil the possible source of these pathogens in our settings and to impede their further spread, strict infection control measures were implemented. This comprised of extensive environmental and disinfectant sampling and active surveillance for possible carriage of pathogens in the doctors and nursing staff. However, after investigating the source of these pathogens, it was found that, all the patients admitted to NICU had been referred from some other hospitals in a nearby city, in need of tertiary level care. They were probably already colonized by MDRO at the time of admission and had been exposed to therapy with multiple antibiotics. Hence, the source could not be eliminated; however, stringent infection control precautions were instituted to prevent their spread in the NICU environment. Moreover, it created another constant challenge for surveillance on the import of these infections from outside on regular basis.
Although we have not assessed individual risk factors for development of VAP in the present study, the analyzed risk factors may provide deeper insights in the pathogenesis caused by these pathogens.
5.1. Conclusions
Repeated isolation of MDR Acinetobacter baumannii along with other resistant phenotypes of gram negative bacteria is a cause of concern for any newly established NICU setup. Long term use of reserved antibiotics leads to selection pressure, resulting in establishment of relatively less common environmental opportunists as new, more troublesome pathogens. It may further complicate the treatment decisions and lengthen the hospital stay and associated morbidity. Hence, strict infection control measures and rational use of reserved antibiotics should be practiced to overcome the unforeseen complications.