1. Background
Malaria is an infectious disease affecting humans worldwide and the most important disease in tropical regions that their manifestations are mostly emerged as ague (1, 2). Malaria is caused by a parasitic protozoan called Plasmodium with an estimated mortality rate of one million people annually and it is estimated that half of the world's population are at risk for this disease, of which about 78% are in Africa, 15% in the South-East Asia, and 5% in the Eastern Mediterranean (3, 4). The asexual stages of the parasite are responsible for all clinical symptoms and physio-pathological damage to the patient (5). Malaria can lead to respiratory distress, metabolic acidosis, hypoglycemia and failure in some organs such as liver, kidney and spleen (6). Current strategies to combat malaria are use of appropriate drugs, vector control and health education services (7-9). Plasmodium berghei species cause malaria in rodents (10). Study on rodent malaria can be an appropriate model for human malarial infection (11). Chloroquine and pyrimethamine are two examples of chemical drugs used to treat malaria (12, 13). Although these two drugs are widely used to treat malaria, increased resistance to them is a big problem to overcome this disease (14).
2. Objectives
The current study aimed to compare the effect of sulfadoxine-pyrimethamine with pyrimethamine and evaluate the efficiency of the two drugs to treat Plasmodium berghei infection in mice.
3. Methods
3.1. Parasite
Plasmodium berghei NICD (national institute for Communicable diseases) strain sensitive to chloroquine was obtained from the department of parasitology and mycology, School of Medicine, Isfahan University of Medical Science, Isfahan, Iran. The parasite was cryopreserved in liquid nitrogen and passaged several times in laboratory mice before the experiment (15).
3.2. Mice
In the current study, male Swiss Webster mice, 20 - 25 grams weight and 8 - 12 weeks old age were used. The use of mice was confirmed by the university ethics committee (UEC) of Isfahan University of Medical Sciences, Isfahan, Iran.
3.3. Drugs
Pyrimethamine, hydroxychloroquine and sulfadoxine-pyrimethamine were purchased from Biogen Company (India); drugs were used in the current study. Formore solubility, the drugs were placed on a magnetic stirrer at a speed of 250 g, at 0°C for 24 hours.
3.4. Evaluation of the Drug Effectiveness
A total of 36 mice were used in the experiment. The mice were randomly divided into four groups of nine. Each of them was infected by intraperitoneally injection of 106 Plasmodium berghei-infected red blood cells (RBC). One group was considered as positive control and two other ones received each one of the drugs and the group four was the negative control. The mice were treated according to the protocol described by Ryley and Peters (1970) (16). Twenty-four hours after the injection, treatment was started by intraperitoneally injection of 2 mg/kg/day of each drug for four days. On days 4, 7, 14, 21 and 28, a blood smear was prepared for each mouse, stained with Giemsa and the number of infected RBCs was calculated at 10,000 counted RBCs under optical microscopy according to the following formula:
Moreover, the survival time of the mice was recorded during 28 days and the average was calculated for each of the groups.
3.5. Statistical Analysis
Data were analyzed by IBM SPSS statistics software (ver. 20) and using a one-way ANOVA.
4. Results
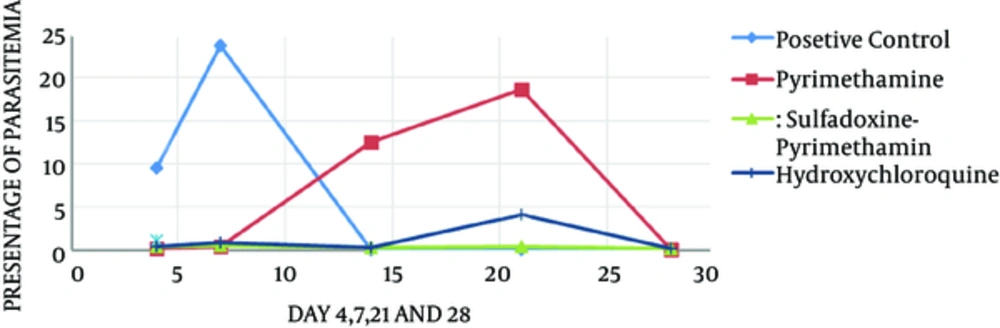
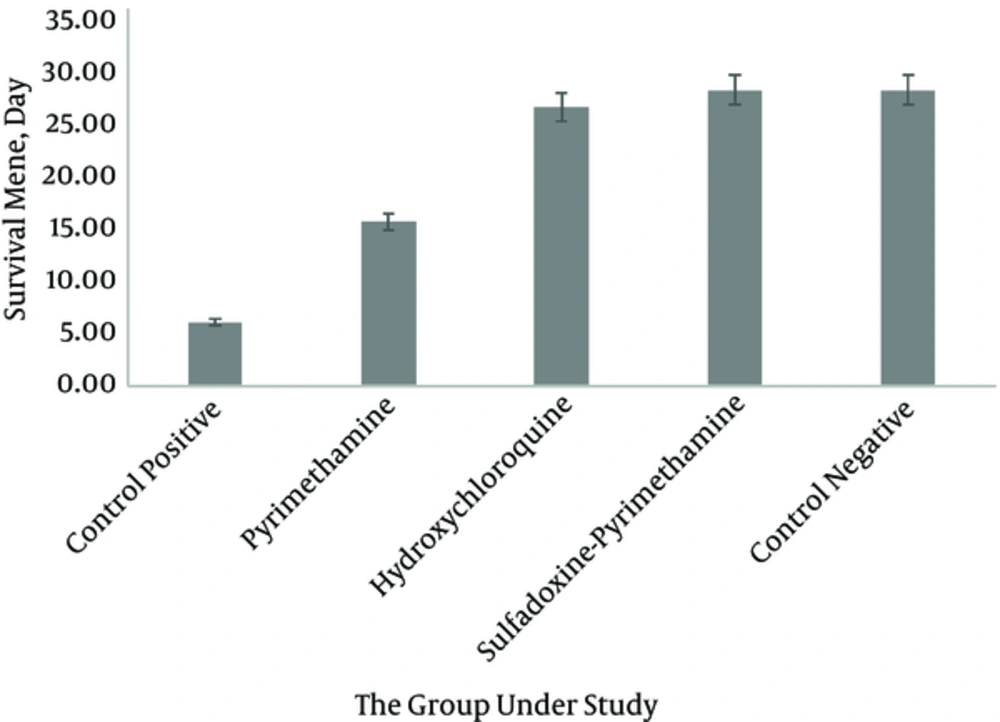
Parasitemia in the positive control group showed an increase on days four and seven at the levels of 5.9% and 23.7%, respectively, which was statistically significant compared with the other groups (P < 0.05) (Figure 1). In the current study, parasitemia remarkably decreased in the group receiving sulfadoxine-pyrimethamine compared with the group receiving only pyrimethamine (P < 0.05). In addition, the mortality rate in the mice treated with sulfadoxine-pyrimethamine was lower than those of the other groups (P < 0.05) (Figure 2). The survival time in the group treated with sulfadoxine-pyrimethamine was 28 days on average and in the group treated with pyrimethamine as well as the hydroxychloroquine were 15.56 and 26.44 days, respectively.
5. Discussion
Nowadays, great efforts are made to control malaria but it is still responsible for killing a large number of people, especially children under five years in the underdeveloped and developing countries (17). The world health organization (WHO) recommended chloroquine and pyrimethamine in the endemic areas for prophylaxis to reduce children mortality rate (18, 19). The first line drugs to treat all types of malaria are chloroquine and pyrimethamine, but extensive failure of treatment with these drugs is currently on the rise (20). Despite the widespread resistance to chloroquine as the first line drug to treat malaria, drug resistance is a major health problem worldwide and the affected countries should plan a new policy for malaria treatment; therefore, the selection of an accessible and affordable anti-malarial drug is still not known to replace chloroquine (21). Sulfadoxine-pyrimethamine in Africa and several countries are proposed as an alternative to chloroquine (22). Countries with a high prevalence of drug resistance increasingly use synergistic drugs such as sulfadoxine and pyrimethamine (sulfadoxine-pyrimethamine) to treat malaria and somehow prevent the spread of drug resistance (23). The administration of sulfadoxine-pyrimethamine provided a low-cost and suitable option for therapeutic aims against malaria in Africa; therefore in a study, a combination of sulfadoxine-pyrimethamine with chloroquine reduced parasitemia and fever in patients (24). Furthermore, another study conducted in Uganda revealed that the combination of sulfadoxine-pyrimethamine with chloroquine can be drug of choice as a temporary replacement for the first-line treatment of malaria (25). In another study conducted in Kampala, amodiaquine combined with sulfadoxine-pyrimethamine effectively reduced the parasitemia (26). In another study, the effect of ciprofloxacin, a second generation antibiotic, combined with amodiaquine was examined in mice infected with chloroquine-resistant Plasmodium spp. In the recent study, animals were treated up to 21 days and although treatment with amodiaquine alone suppressed parasitemia in the infected mice; ciprofloxacin combined with amodiaquine was much more effective than amodiaquine alone (27). In another study, the effect of artemisinin-based combination therapy on Plasmodium vivax led to reduction of parasitemia and shortened the duration of fever compared to chloroquine. In addition, artemisinin-based combination therapy reduced the relapse of the disease (28). Another study also compared the effect of two drugs chloroquine and ciprofloxacin in which the combination of 160 mg/kg ciprofloxacin with chloroquine showed a better therapeutic effect than each of them alone (29). In the present study the therapeutic effect of the combinatory drug sulfadoxine-pyrimethamine was far more than those of pyrimethamine and hydroxychloroquine alone.
According to the results of the other aforementioned studies and the current one, it can be concluded that the combinatory drugs are more effective than single ones (the present study) and most importantly can prevent relapse (28) and also reduce the probability of drug resistance (23). As a result, the article suggests that malaria treatment strategy shifts completely from the monotherapy and combination drugs should be used for this purpose, especially in endemic areas.