1. Background
The newly transformed coronavirus has caused a worldwide disaster since December 2019, as well as millions of deaths until now. Severe acute respiratory syndrome (SARS) is the most prevalent phenomenon of the disease. Therefore, COVID-19 is caused by SARS-CoV-2. This is the third time (SARS, MERS, and COVID-19) that a coronavirus has caused an outbreak. The first outbreak (SARS-CoV-1) was in China in 2002 - 2004 (1). To date, 442 million confirmed cases of COVID-19, with 5.7 million deaths, have been reported (2). The pathogenesis of SARS-CoV-2 is related to a receptor called angiotensin-converting enzyme 2 (ACE2), causing the virus to enter human cells by connecting to virus spike protein (S1) (3). Therefore, every organ that expresses ACE2 on its cell surface could be infected by SARS-CoV-2. ACE2 catalyzes and converts angiotensin II into angiotensin 1 - 7, helping maintain blood pressure homeostasis by regulating the renin-angiotensin system (RAS), vasodilation, and anti-inflammation (4). The manifestations of SARS-CoV-2, such as acute respiratory distress syndrome (ARDS), can be explained by ACE2 expression in the human alveolar cells. The density of ACE2 receptors is higher in smokers, and the severity of COVID-19 is also higher (5).
Thyroid gland follicular cells have ACE2 on their surface (6). Thyroid hormones have a vital role in modulating RAS. In other words, rising thyroid hormones (such as hyperthyroidism) can increase RAS (7). In RAS, renin cleaves angiotensinogen to form angiotensin I; then, ACE turns angiotensin I to angiotensin II by removing 2 amino acids (8). The regulation of RAS by thyroid hormones and the classic RAS axis can be proof of ACE2 receptors expressed on thyroid gland cells.
On the other hand, the immune response to SARS-CoV-2 infection leads to the release of inflammatory cytokines, especially interleukin 6 (IL-6), leading to thyroid hormone dysfunction (9, 10). According to several case reports, COVID-19 causes acute and subacute thyroiditis and Grave’s hyperthyroidism (11-14).
The present study is a retrospective case-control and cross-sectional study on 191 COVID-19 patients hospitalized in Amir-al-Momenin Hospital affiliated with Islamic Azad University of Medical Sciences, Tehran, Iran, and 179 non-COVID-19 individuals as the control group. This study investigated the thyroid hormone status in SARS-CoV-2 infection in comparison with the control group, as well as the relationship between thyroid hormones and different aspects of COVID-19, including underlying diseases, duration of hospitalization, duration of symptoms before hospitalization, SpO2, and respiratory rate (RR). The patients were divided into 2 groups with and without normal thyroid function by thyrotropin levels and compared with each other in different aspects of COVID-19. Also, we compared thyroid hormone levels in the patient group with different underlying diseases to show the status of thyroid function in COVID-19 infection.
2. Methods
This is a single-center retrospective case-control, cross-sectional study on 191 COVID-19 patients hospitalized at Amir-al-Momenin Hospital affiliated with Islamic Azad University of Medical Sciences, Tehran, Iran, and 179 non-COVID-19 outpatient individuals without infection or severe disease; they had a lab test for a checkup from 30 March 2020 to 6 May 2021. All the COVID-19 patients were positive for SARS-CoV-2 infection, based on reverse transcriptase-polymerase chain reaction (RT-PCR) of nasopharyngeal and oropharyngeal swab specimens. First, 217 COVID-19 patients were chosen for this study, and 26 were excluded due to the inclusion and exclusion criteria. Inclusion criteria were age more than 18 years old and hospitalization for COVID-19. The patients with a previous history of thyroid problems and taking medication affecting the thyroid gland (and glucocorticoids) and pregnancy were excluded; therefore, the number of participants decreased to 191 COVID-19 patients and 179 non-COVID-19 individuals. Thyroid function blood tests and clinical examinations were performed for each patient on the first day of admission. Demographic information, including gender, age, coexisting conditions, duration of hospitalization, duration of symptoms before hospitalization, SpO2, and RR, were gathered from patients’ records. The study followed the principles of the Declaration of Helsinki. The Ethics Committee of Islamic Azad University of Tehran Pharmacology Sciences approved this study (code: IR.IAU.PS.REC.1400.436).
The blood sample was taken from all COVID-19 patients hospitalized for thyrotropin, total thyroxine (TT4), and total triiodothyronine (TT3). The patients whose blood samples were taken on the first day of hospitalization were selected. The analyses were performed by the chemiluminescence immunoassay method using an ADVIA centaur XP immunoassay system (Siemens®) at the Amir-Al-Momenin Hospital laboratory. In addition, SpO2 was measured by a pulse oximeter, and RR was calculated as the number of inhales and exhales per minute. The normal range for thyrotropin, TT3, and TT4 was 0.45 to 4.5 mIU/L, 80 to 180 ng/dL (1.2 - 2.7 nmol/L), and 4.5 to 12.6 μg/dL (58 - 160 nmol/L).
First, the thyroid hormone status was compared between the patient and control groups to determine whether the correlation was significant. In the next step, we compared different aspects of COVID-19 infection, including duration of hospitalization, duration of symptoms before hospitalization, RR, SpO2, and mortality with thyrotropin, TT3, and TT4 levels. Otherwise, we also observed the effect of different underlying diseases on thyroid function. On the other hand, the patients were divided into 2 groups with and without normal thyrotropin levels. The number of patients with proper thyrotropin, TT3, and TT4 levels was 189, 63, and 85. In the control group, 179, 73, and 111 participants had thyrotropin, TT3, and TT4 levels, respectively.
All the data were analyzed using SPSS version 26 (SPSS Inc, Chicago, IL, USA). Mean ± SD, frequency of the quantitative data, and frequencies of qualitative data were calculated. The data comparison was made as a 2-sided P value, and a P value of less than 0.05 was defined as statistically significant. Overall, the Kolmogorov-Smirnov test was applied to test the normality of data distribution. The Mann-Whitney U test was performed to calculate the P value for age, thyrotropin, TT3, and TT4 in both patient and control groups, as well as to compare the mortality rate with thyroid function. The Fisher exact test was used to compare gender in both patient and control groups. Then, to compare quantitative data (including thyrotropin, TT3, and TT4 levels) with the mean ± SD of age, duration of hospitalization, SpO2, and RR, the Spearman rho correlation and independent t-test were used. The Spearman rho correlation and Mann-Whitney U test were used for the duration of symptoms before hospitalization. To compare each underlying disease with thyroid hormones, an independent t-test was performed, and the Levene test was used for equality of variances.
3. Results
Of the 191 COVID-19 patients, 98 (51.3%) were male, and of the 179 non-COVID-19 individuals, 61 (34.1%) were male (P = 0.001). The mean ± SD age of the control group was 43 ± 13 years (minimum, 20; maximum, 81), and the mean age of patients was 64 ± 15 years (minimum, 19; maximum, 109; P = 0.000). The age of the patients was significantly associated with thyroid hormones (P < 0.05; 0.25 < r < 0.5; Table 1). The thyrotropin level was lower in the patient group than in the control group (1.34 ± 1.29 vs. 2.21 ± 1.99; P < 0.001). Despite the significant difference in thyrotropin levels between the patient and control groups, the thyroid hormones (T3 and T4) were not meaningfully different (P > 0.05) between the 2 groups (Table 2).
| Variables | Mean ± SD | Spearman Rho (P Value) vs. Thyrotropin | Spearman Rho (P Value) vs. T3 | Spearman Rho (P Value) vs. T4 |
|---|---|---|---|---|
| Age | 64 ± 15 | -0.013 (0.862) | -0.485 (< 0.001) | -0.310 (0.004) |
| Duration of symptoms | 7 ± 4 | -0.083 (0.256) | 0.053 (0.682) | 0.165 (0.130) |
| Duration of hospitalization | 7 ± 4 | 0.052 (0.477) | -0.013 (0.917) | 0.097 (0.375) |
| SpO2 | 90 ± 6 | 0.074 (0.311) | -0.258 (0.043) | 0.049 (0.660) |
| RR | 22 ± 5 | -0.078 (0.284) | 0.159 (0.213) | -0.133 (0.225) |
Comparison of Different Variables in COVID-19 Patients with Thyroid Hormone Function Status
| Variables | COVID-19 Patients (n = 191) | Non-COVID-19 Individuals (n = 179) | P Value |
|---|---|---|---|
| Gender | < 0.001 | ||
| Male | 98 (51.3) | 61 (34.1) | |
| Female | 93 (48.7) | 118 (65.9) | |
| Age | 64 ± 15 | 43 ± 13 | < 0.001 |
| Thyrotropin | 1.34 ± 1.29 | 2.21 ± 1.99 | < 0.001 |
| T3 | 1.08 ± 1.46 | .86 ± .96 | 0.237 |
| T4 | 8.46 ± 2.42 | 7.96 ± 1.94 | 0.115 |
Characteristics of COVID-19 Patients and Non-COVID-19 Individuals a
For COVID-19 patients, the mean day of hospitalization was 7 ± 4 days, and the mean day of symptoms before hospitalization was also 7 ± 4 days. The mean SpO2 of patients was 90% ± 6%. The mean RR of the patients was 22 ± 5 per minute. The correlation of thyrotropin with SARS-CoV-2 infection aspects (including duration of hospitalization, duration of symptoms before hospitalization, RR, SpO2, and mortality rate) was not significant. However, the relationship of thyroid hormones (T3 and T4) with COVID-19 aspects demonstrated that only the TT3 status was meaningfully related to the SpO2 level; by increasing the level of TT3, the SpO2 level decreased significantly (P < 0.05; Table 1).
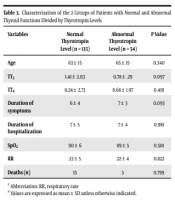
The two groups of patients (normal and abnormal thyrotropin levels) were also compared in different features. The mean ± SD thyrotropin level of patients with normal and abnormal thyroid levels was 1.56 ± 0.99 and 0.78 ± 1.72, respectively; 135 (70.7%) patients had a normal range of thyrotropin. The mean age of patients with normal thyroid function was lower than the other group (63 years old vs. 65 years old). The TT4 level was higher in patients with abnormal thyrotropin (8.24 ± 2.73 vs. 8.68 ± 1.97); however, the TT3 level was lower (1.41 ± 2.03 vs. 0.78 ± 0.29). The duration of symptoms before admission was less in the group with normal thyroid function tests (6 ± 4 vs. 7 ± 3 days). The duration of hospitalization and RR were the same in both groups (7 ± 5 vs. 7 ± 4 days; 22 ± 5 vs. 22 ± 4 per minute). The mortality of patients with normal thyroid was more than the other group (P > 0.05; Table 3)
| Variables | Normal Thyrotropin Level (n = 135) | Abnormal Thyrotropin Level (n = 54) | P Value |
|---|---|---|---|
| Age | 63 ± 15 | 65 ± 15 | 0.340 |
| TT3 | 1.41 ± 2.03 | 0.78 ± .29 | 0.097 |
| TT4 | 8.24 ± 2.73 | 8.68 ± 1.97 | 0.419 |
| Duration of symptoms | 6 ± 4 | 7 ± 3 | 0.093 |
| Duration of hospitalization | 7 ± 5 | 7 ± 4 | 0.981 |
| SpO2 | 90 ± 6 | 89 ± 5 | 0.510 |
| RR | 22 ± 5 | 22 ± 4 | 0.822 |
| Deaths (n) | 15 | 5 | 0.799 |
Characterization of the 2 Groups of Patients with Normal and Abnormal Thyroid Functions Divided by Thyrotropin Levels
For COVID-19 patients, the most common underlying diseases were hypertension (48.7%), diabetes mellitus (36.1%), ischemic heart disease (26.2%), and hyperlipidemia (11.5%). None of these diseases were significantly related to thyroid function (P > 0.05). Among other underlying disorders, only patients with asthma (n = 7; 3.7%) were significantly different by thyrotropin status (P = 0.001). Asthma did not have a significant relationship with the status of other thyroid hormones (P > 0.05; Table 4).
| Underlying Diseases | Frequency (%) | P Value (Thyrotropin) | P Value (T3) | P Value (T4) |
|---|---|---|---|---|
| Hypertension | 93 (48.7) | 0.189 | 0.115 | 0.823 |
| Diabetes mellitus | 69 (36.1) | 0.469 | 0.273 | 0.990 |
| Ischemic heart diseases | 50 (26.2) | 0.632 | 0.315 | 0.483 |
| Hyperlipidemia | 22 (11.5) | 0.489 | 0.615 | 0.266 |
| Cerebrovascular accident | 8 (4.2) | 0.322 | 0.796 | 0.497 |
| Asthma | 7 (3.7) | 0.001 | - | 0.995 |
| Alzheimer disease | 5 (2.6) | 0.940 | - | - |
| Fatty liver | 5 (2.6) | 0.054 | - | - |
| Benign prostate hyperplasia | 5 (2.6) | 0.572 | 0.673 | 0.743 |
| Anemia | 5 (2.6) | 0.650 | 0.547 | - |
| Depression | 4 (2.1) | 0.283 | - | 0.635 |
| Chronic kidney disease | 4 (2.1) | 0.201 | 0.709 | 0.721 |
Effects of Underlying Diseases on Thyroid Hormone Function Status in COVID-19 Patients
4. Discussion
This retrospective, cross-sectional, case-control study was conducted on 191 COVID-19 patients and 179 non-COVID-19 individuals as the control group to investigate the probable relationship between thyroid function (thyrotropin, T3, and T4) and SARS-CoV-2 infection. SARS-CoV-2 infection may affect the pituitary-thyroid axis, causing possible secondary hypothyroidism (15). Direct or indirect pituitary gland damage by SARS-CoV-2 may upregulate thyrotropin secretion, causing a reduction in thyrotropin levels (16). Also, an analysis of the thyroid gland’s surgical sample has shown that thyroid follicular cells express ACE2, which could be a target for SARS-CoV-2 (17). Many case reports have suggested the relationship of COVID-19 with subacute thyroiditis that occurs 16-36 days after infection (18-20). Despite Hashimoto thyroiditis and myxedema coma cases in COVID-19 patients, the occurrence of thyroid autoimmunity in COVID-19 is still unclear (21, 22). These studies suggest that a transient dysfunction of the thyroid gland may occur in COVID-19 patients (23).
Our study demonstrated that thyrotropin levels were significantly lower in COVID-19 patients than in the control group; however, the levels of thyroid hormones were not significantly different between the two groups. In a study by Chen et al. in China, the thyrotropin level and TT3 were significantly different between 50 COVID-19 patients and the control group (24). On the other hand, our results showed a nonsignificant difference in the T4 level between the two groups, which is in line with the results of Chen et al. (24). In our study, the severity of the disease was not related to thyroid function, but SpO2 was significantly associated with T3 levels. However, the severity of the disease, including SpO2, was significantly related to thyrotropin and T3 levels in the study by Chen et al. (24). Overall, the mean age in the study by Chen et al. (24) was 16 years less than ours (24). In another study by Beltrao et al. in Brazil, the mortality of 245 COVID-19 patients was not significantly related to thyrotropin and FT4 levels, which is in line with our study (25). Another study by Malik et al. in Pakistan demonstrated that in 48 patients, the thyrotropin and TT3 levels were significantly different from the control group (26). Most of the existing studies on thyroid function in COVID-19 patients investigated the severity of the disease and thyroid function.
In contrast, Gao et al. proved that the FT3 was significantly different in 100 COVID-19 patients than in the control group and was a predictive factor for the mortality rate; however, the thyrotropin and T4 levels were not different in the two groups (27). Gao et al. also demonstrated that the clinical deterioration of COVID-19 patients was associated with lower levels of thyrotropin and FT3, while our study showed that only SpO2 was deteriorated by the T3 level (27). Lui et al. performed a study with the same number of patients as ours and could not find a significant relationship between the age of the patients and thyrotropin levels (28). However, in our study, the thyrotropin level was associated with age (28). Unexpectedly, we could not find any relationship between thyrotropin level and different aspects of COVID-19. Altogether, although the results of studies on thyroid function in COVID-19 patients are inconsistent, theoretically, thyroid function may be a predictive factor in COVID-19 patients. Our study differs from other studies because it was conducted over a longer period of time (about one year), which contained different peaks of the disease in Iran.
The evaluation of the association of underlying diseases with thyroid function in our study represented that asthma was significantly associated with the thyrotropin level. However, one study did not show any association between thyroid function and asthma (29). Another study on 78 men with asthma demonstrated that FT3 was associated with asthma but not thyrotropin (30). The relationship between asthma and thyroid function is unclear, while thyroidectomy in rats showed susceptibility to developing asthma (31). Meanwhile, the number of COVID-19 patients with asthma (n = 7; 3.7%) was small to determine whether the statistics were reliable.
If the anti-thyroid peroxidase antibody level and thyroid sonography had been performed, the results of this study could have been more precise. Nevertheless, this was a retrospective study, and not much more information could be gained from patients.
4.1. Conclusions
The thyrotropin level was lower in COVID-19 patients than in controls. The T3 level can predict the SpO2. The thyroid gland may theoretically be affected by SARS-CoV-2 infection. Among the underlying diseases and thyroid function in this study, asthma was associated with thyroid dysfunction. However, due to the limitations of this study, we recommend cohort studies with larger sample sizes, taking the anti-thyroid peroxidase antibody level and thyroid sonography for more relevant and reliable results.