1. Background
Urinary tract infections (UTIs) are caused by a wide range of pathogens, constituting one of the most important public health problems worldwide (1). UTIs can affect both genders in all age groups. However, some individuals are at higher risk. In general, adult women are 30 times more likely to develop UTI, with 40% of all of them experiencing it at some point in their lives (2, 3). UTIs are reported to be the second most commonly treated infection in primary care and are the most common infection seen in a hospital setting, encompassing 40% of all hospital-acquired infections (4, 5). Microbial agents can infect any part of the genitourinary system and are clinically categorized as complicated or uncomplicated. In healthy patients and in the absence of structural abnormalities, uncomplicated UTIs are further differentiated as either upper or lower, typically seen as pyelonephritis and cystitis, respectively (6). These patients typically present with dysuria, urgency, urinary frequency, fever, and flank pain (7). Complicated UTIs are those seen during pregnancy, in patients with renal failure or transplantation, or in immunosuppressed patients but are most commonly associated with indwelling catheters (8). Diagnosis is based on signs and symptoms of infection and urine analysis (UA), with urine culture (UC) typically being reserved for complicated UTIs (9). The treatment of choice in UTIs depends on whether it is complicated or not, with antibiotics such as ciprofloxacin and ampicillin being the most commonly prescribed (2, 10). However, treatment is complicated with increasing rates of antibiotic resistance and the emergence of multidrug-resistant pathogens. Thus, it is important to evaluate the frequency of these resistances in different microbial agents, to further understand their mechanism and to provide adequate treatment (11).
2. Objectives
In this study, the frequency of bacterial agents and the pattern of their antibiotic resistance were evaluated in UTI patients referred to teaching hospitals in Khorramabad in 2021.
3. Methods
3.1. Study Design and Participants
This cross-sectional study was conducted at Shahid Rahimi and Shohada-ye Ashayer hospitals in Khorramabad, Iran, in 2021. The sampling method was a census, and all patients who met the inclusion criteria were recruited. The inclusion criteria for this study were: (1) diagnosis of UTI in 2021, (2) recorded Urine Culture and antibiogram, and (3) age 16 years or older. The patients were excluded if their medical records were incomplete or did not consent to participate in the study. Finally, 250 patients were enrolled in the study.
3.2. Data Collection
After obtaining written consent, demographic information including age, gender, place of residence, occupation, educational level, history of underlying diseases, and history of antibiotic use as well as information relating to the type of pathogen and the antibiotic resistance and sensitivity, were collected through reports of UC results and antibiograms recorded in the patient’s medical files and registered into a researcher-made checklist.
3.3. Data Analysis
The collected data were analyzed by Stata software (version 14) using chi-square and independent t-tests. Furthermore, multivariable logistic regression was used to investigate the prevalence of pathogens and their antibiotic resistance, with results being reported at a significance level of 5%.
3.4. Ethical Considerations
This study was conducted with the permission of the Research Ethics Committee of Lorestan University of Medical Sciences with the ethical code IR.LUMS.REC.1401.076. Written, informed, and voluntary consent was obtained from all participants. All details regarding the medical records of the patients were kept confidential. Hence, the principles of medical ethics, The Helsinki Declaration, were observed.
4. Results
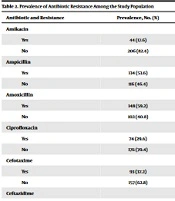
The study population consisted of 163 women (65.2%) with a mean age of 54.52 ± 10.12 and 87 men (34.8%) with a mean age of 51.87 ± 12.01. Data regarding the history of the underlying disease and recent antibiotic use were recorded, with 18.4% and 96.4% having a positive history, respectively. Table 1 shows the frequency distribution of pathogens reported from the urine culture in the study population. The highest reported frequency of pathogens was related to Escherichia coli (41.20% or 103 patients), followed by Staphylococcus saprophyticus (20.80% or 52 patients) and Klebsiella pneumoniae (18.40% or 46 patients). The least frequently reported pathogens were Citrobacter and Proteus, with each consisting of 2% of the study population. Antibiotic resistance in these pathogens is shown in Table 2, with amoxicillin and ampicillin being the most frequently reported (59.2% and 53.6%, respectively). The least frequently reported antibiotic resistance among all pathogens was imipenem (11.6%), followed by amikacin (17.6%). Tables 3 and 4 display the frequency of resistance of different antibiotics by gender and age, respectively, and the chi-square test was used to determine statistical significance (P-value of < 0.05). This study also showed that there was no significant relationship between antibiotic resistance and gender in the different drug classes. Additionally, antibiotic resistance among patients was also evaluated based on the history of the underlying disease and was found to be insignificant (P-value > 0.05) among the different drug classes (Table 5).
| Pathogen | Prevalence, No. (%) |
|---|---|
| Escherichia coli | 103 (41.2) |
| Staphylococcus saprophyticus | 52 (20.8) |
| Klebsiella pneumoniae | 46 (18.4) |
| Enterobacter | 24 (9.6) |
| Staphylococcus epidermis | 8 (3.2) |
| Proteus | 5 (2) |
| Citrobacter | 5 (2) |
| Others | 7 (2.8) |
| Total | 250 (100) |
Frequency Distribution of Pathogens Seen in UCs
| Antibiotic and Resistance | Prevalence, No. (%) |
|---|---|
| Amikacin | |
| Yes | 44 (17.6) |
| No | 206 (82.4) |
| Ampicillin | |
| Yes | 134 (53.6) |
| No | 116 (46.4) |
| Amoxicillin | |
| Yes | 148 (59.2) |
| No | 102 (40.8) |
| Ciprofloxacin | |
| Yes | 74 (29.6) |
| No | 176 (70.4) |
| Cefotaxime | |
| Yes | 93 (37.2) |
| No | 157 (62.8) |
| Ceftazidime | |
| Yes | 98 (39.2) |
| No | 152 (60.8) |
| Gentamicin | |
| Yes | 78 (31.2) |
| No | 172 (68.8) |
| Ceftriaxone | |
| Yes | 97 (38.8) |
| No | 153 (61.2) |
| Nalidixic acid | |
| Yes | 109 (43.6) |
| No | 141 (56.4) |
| Kanamycin | |
| Yes | 83 (33.2) |
| No | 167 (66.8) |
| Imipenem | |
| Yes | 29 (11.6) |
| No | 221 (88.4) |
Prevalence of Antibiotic Resistance Among the Study Population
| Antibiotic and Gender | Prevalence of Resistance | P-Value |
|---|---|---|
| Amikacin | 0.133 | |
| Male | 11 | |
| Female | 33 | |
| Ampicillin | 0.866 | |
| Male | 46 | |
| Female | 88 | |
| Amoxicillin | 0.686 | |
| Male | 53 | |
| Female | 96 | |
| Ciprofloxacin | 0.217 | |
| Male | 30 | |
| Female | 44 | |
| Cefotaxime | 0.861 | |
| Male | 33 | |
| Female | 60 | |
| Ceftazidime | 0.606 | |
| Male | 36 | |
| Female | 62 | |
| Gentamicin | 0.595 | |
| Male | 29 | |
| Female | 49 | |
| Ceftriaxone | 0.735 | |
| Male | 35 | |
| Female | 62 | |
| Nalidixic acid | 0.175 | |
| Male | 43 | |
| Female | 66 | |
| Kanamycin | 0.595 | |
| Male | 27 | |
| Female | 56 | |
| Imipenem | 0.97 | |
| Male | 10 | |
| Female | 19 |
The Statistical Relationship Between Gender and Antibiotic Resistance
| Antibiotic and Age (y) | Prevalence of Resistance | P-Value |
|---|---|---|
| Amikacin | 0.053 | |
| < 50 | 22 | |
| > 50 | 22 | |
| Ampicillin | 0.098 | |
| < 50 | 62 | |
| > 50 | 72 | |
| Amoxicillin | 0.056 | |
| < 50 | 64 | |
| > 50 | 84 | |
| Ciprofloxacin | 0.066 | |
| < 50 | 33 | |
| > 50 | 41 | |
| Cefotaxime | 0.132 | |
| < 50 | 39 | |
| > 50 | 54 | |
| Ceftazidime | 0.07 | |
| < 50 | 42 | |
| > 50 | 56 | |
| Gentamicin | 0.585 | |
| < 50 | 30 | |
| > 50 | 48 | |
| Ceftriaxone | 0.056 | |
| < 50 | 42 | |
| > 50 | 55 | |
| Nalidixic acid | 0.64 | |
| < 50 | 41 | |
| > 50 | 68 | |
| Kanamycin | 0.383 | |
| < 50 | 33 | |
| > 50 | 50 | |
| Imipenem | 0.061 | |
| < 50 | 15 | |
| > 50 | 14 |
The Statistical Relationship Between Age and Antibiotic Resistance
| Antibiotic and Underlying Disease | Prevalence of Resistance | P-Value |
|---|---|---|
| Amikacin | 0.967 | |
| Yes | 8 | |
| No | 36 | |
| Ampicillin | 0.128 | |
| Yes | 20 | |
| No | 114 | |
| Amoxicillin | 0.082 | |
| Yes | 22 | |
| No | 126 | |
| Ciprofloxacin | 0.099 | |
| Yes | 9 | |
| No | 65 | |
| Cefotaxime | 0.076 | |
| Yes | 10 | |
| No | 83 | |
| Ceftazidime | 0.094 | |
| Yes | 12 | |
| No | 86 | |
| Gentamicin | 0.125 | |
| Yes | 10 | |
| No | 68 | |
| Ceftriaxone | 0.197 | |
| Yes | 14 | |
| No | 83 | |
| Nalidixic acid | 0.499 | |
| Yes | 18 | |
| No | 91 | |
| Kanamycin | 0.659 | |
| Yes | 14 | |
| No | 69 | |
| Imipenem | 0.735 | |
| Yes | 6 | |
| No | 23 |
The Statistical Relationship Between Underlying Disease and Antibiotic Resistance
5. Discussion
The inappropriate use of antibiotics for the treatment of infections can impose negative effects on public health economically and lead to drug resistance. Thus, it is crucial to incessantly monitor antimicrobial resistance patterns in all regions (12). In this cross-sectional study, the prevalence of microbial pathogens amongst UTI patients as well as their antibiotic resistance condition was evaluated. The results showed that E. coli was the most commonly reported pathogen, which was consistent with other studies conducted showing this microorganism to be the most common cause of UTI. The prevalence of this bacterium ranges from 10% to 73.7% in different populations (13-16). Congruent with the results of other studies (12), Staphylococcus was the most frequent Gram‑positive bacteria.
Additionally, in this study, the highest rate of resistance in both sexes, regardless of the bacterial strains, was reported to be against amoxicillin (59.2%) followed by ampicillin (53.6%), which were consistent with the results of Setu et al. (17) and Abedi Samakoosh et al. studies (18). Consistent with the results of Mortazavi-Tabatabaei et al. (19) and Hossain et al. (20) studies, the lowest rate of resistance was seen regarding imipenem (11.6%) and amikacin (17.6%). It should be noted that the majority of the studied subjects (96.4%) had a history of recent antibiotic use. As a previous use of antibiotics can affect the prevalence and patterns of antibiotic resistance among patients with UTI (21), the findings of this study should be interpreted considering the fact that there was a positive history of antibiotic use in most subjects.
Global studies indicate that the causes and resistance patterns of urinary infections have changed (22). Therefore, identifying the bacterial agents that cause UTIs and using appropriate and effective antibiotics to eliminate them is one of the practical applications of dealing with these infections and preventing their sequelae. Moreover, due to the constant alteration of antibiotics, the high rates of microbial resistance to common drugs incur exorbitant treatment costs (23, 24). The increasing diversity of resistance to antibiotics can be justified by variations in the regional use of antibiotics. Inappropriate and excessive use of antibiotics leads to resistance; therefore, reducing the prescription of specific antibiotics can lead to a reduction in microbial resistance (25, 26). The high rate of resistance of bacteria to antibiotics in the present study and other studies conducted in Iran can be attributable to the indiscriminate prescription of antibiotics and their self-administration without a prescription. Additionally, other risk factors, such as recurrent UTIs and urinary tract abnormalities, affect the development of antibiotic resistance. In the treatment and management of UTIs, empirical antibiotics are given prior to the antibiogram results, which, beneficial as it may be for rapid relief, may also increase resistance long term. In this study, the least frequent resistance rate was seen regarding imipenem and amikacin; thus, they can be useful as empirical therapy in UTIs.
5.1. Limitations
One limitation of the present study was the use of data from a single center and a lack of country-wide generalization of resistance to accurately analyze their rates and patterns. However, in this study, different potential contributing demographic factors were analyzed to assist in the understanding of microbial resistance mechanisms. Another limitation was the small sample size, and the study is based on a census basis. Therefore, it is recommended to conduct similar studies with a random sampling of a larger population pool so that the results can be more generalizable.
5.2. Conclusions
In this study, consistent with the literature, a high rate of resistance was observed against the commonly used antibiotics. It is crucial to prescribe antibiotics methodically to prevent bacterial resistance to antimicrobial agents and reserve those with relatively infrequent resistances, such as imipenem and amikacin, for confirmed severe infections. Additionally, considering the importance of understanding antibiotic resistance patterns, epidemiological data from antibiograms are necessary for the treatment and management of UTIs.