1. Background
Escherichia coli is a normal inhabitant of the gastrointestinal tract of humans and animals and is commonly recovered from vegetation, soil, and water (1). It is one of the most frequent etiologies of nosocomial and community-acquired infections worldwide (1). The bacterium is the most frequent cause of urinary tract infections and a major etiology of bacteremia, sepsis, surgical site infections, and gastroenteritis in both outpatients and inpatients. It is also a common cause of meningitis in newborns and immunocompromised individuals (1). Emerging resistance of the organism to different types of antibiotics is a global concern, complicating therapy for infected patients (1, 2). One of the most important mechanisms in the resistance of the bacterium to antibiotics is the production of extended-spectrum beta-lactamase (ESBL), which makes the bacterium resistant to all Penicillins and extended-spectrum Cephalosporins (1, 2).
The development of antibacterial resistance in pathogens is a dynamic phenomenon that varies over time and across different geographical locations (1, 2). Consequently, repeated and regular assessment of the sensitivity profile of common pathogens in different communities is a main requirement (1, 2). Few well-designed studies have been performed on the antibiotic susceptibility of E. coli in Iranian populations. Most earlier studies in this regard only reported the antibacterial susceptibility of uropathogenic E. coli. Additionally, a significant number of these investigations did not have a strategy to reject contaminated samples. Furthermore, resistance patterns had not been reported in different situations, such as outpatients versus inpatients and community-acquired versus healthcare-associated isolates (3-7).
2. Objectives
This investigation aimed to determine the susceptibility pattern of E. coli strains isolated from patients admitted to three large medical centers in Isfahan, Iran. This research is clinically noteworthy as it helps clinicians prescribe empiric antibiotics for patients with suspected E. coli infections in the area.
3. Methods
3.1. Study Design
This research aimed to report the antimicrobial susceptibility pattern of E. coli in patients with documented bacterial infections who were admitted to three large medical centers in Isfahan City, Iran, according to age category, site of infection, and community/hospital source of infection. The medical centers participating in the survey were Dr. Shariati, Al-Zahra, and Dr. Gharazi hospitals. The laboratories of these hospitals have Quality Credit for microbiological reports from the Iranian Ministry of Health. Determination of contamination, hospital/community source of infection, and the site of infection was done by trained infection control nurses and physicians in the enlisted hospitals (8).
3.2. Bacterial Isolation and Antibiotic Susceptibility Testing
For the detection of microbial agents, samples were collected using aseptic techniques from urine, bloodstream, cerebrospinal fluid, deep collections, draining surgical sites, or bronchoalveolar lavage (BAL) (8). Identification of isolates as E. coli was done by routine conventional tests such as gram staining and biochemical tests (catalase and oxidase tests), reactions on triple sugar iron (TSI) agar, Methyl Red (MR) and Voges-Proskauer (VP) tests, indole production, urease test, citrate utilization, lysine iron agar (LIA) test, and motility.
The sensitivity pattern of E. coli was obtained by the disk diffusion method according to the Clinical Laboratory Standard Institute (CLSI) recommendations (9). Commercially prepared dehydrated antibiotic discs from MAST, Merseyside, UK, were used. Laboratories assessed the sensitivity of isolates to the following antibiotics: Gentamicin 10 µg or amikacin 30 µg, cefotaxime 30 µg, ceftriaxone 30 µg, ceftazidime 30 µg, cefepime 30 µg, ciprofloxacin 5 µg, trimethoprim-sulfamethoxazole 1.25/23.75 µg, and imipenem 10 µg or meropenem 10 µg.
Isolates that exhibited resistance to cefotaxime or ceftazidime underwent screening for ESBL production via the combination disc method as recommended by CLSI. A positive test for ESBL production was indicated by a ≥ 5 mm increase in the inhibition zone diameter for both antimicrobial agents when tested in combination with clavulanate versus the inhibition zone diameter of the agents when tested alone.
3.3. Identification of Contaminated Isolates
Assuming that E. coli has been isolated from a patient with clinical or paraclinical findings of infection at the isolation site, such as fever or focal signs of infection, it is considered a true pathogen. Otherwise, isolates are considered contaminated (8).
3.4. Differentiation of Community from Nosocomial Isolates
If E. coli was isolated from a clinical specimen after 48 hours of admission with new signs of infection, it was considered a nosocomial organism; all other isolates were defined as community-acquired (8).
3.5. Statistical Analysis
Antimicrobial sensitivity, ESBL production, and the hospital/community source of the isolates, in addition to the diagnosis and age group of the infected patients, were prepared using WHONET v 5.6 software. Analysis was done with SPSS Version 18.0. Comparisons of antibiotic susceptibility in different infections, age groups, ESBL production, and the hospital/community source of the isolates were made using chi-square and Fisher exact tests. A P-value of less than 0.05 was considered significant.
4. Results
A total of 1679 E. coli isolates were found, with 25.7% (431) classified as contamination. Of 1248 patients with documented E. coli infections, 45.8% were males, 11.8% were less than 20 years old, and 85.4% were community-acquired. Most E. coli isolates were cultivated from inpatients with urinary tract infections (UTIs) (71.9%), followed by bloodstream infections (15.1%), skin and soft tissue infections (7.8%), and other infections (5%).
Antimicrobial sensitivity of E. coli isolates revealed that the bacterium was more susceptible to Meropenem (98.0%), Imipenem (98.0%), and Amikacin (94.6%), followed by Gentamicin (68.6%), Cefepime (51.9%), Ceftazidime (46.8%), Ceftriaxone (41.3%), Ciprofloxacin (39.5%), Cefotaxime (39.3%), and Trimethoprim-sulfamethoxazole (32.4%). In contrast to Imipenem, which was more effective in patients older than 20 years, the sensitivity of the isolates to Ciprofloxacin was lower in that age group. In addition, E. coli isolates were more susceptible to Ceftazidime in community-acquired infections than in nosocomial infections (Table 1).
| Antibiotic | Age Group | Source of the Infection | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| <20 yrs n/N b | >20 yrs n/N b | P- Value | Odds Ratio (95CI) | Community n/N b | Hospital n/N b | P- Value | Odds Ratio (95 | ||
| ESBL + | 47/147 | 341/1101 | 0.805 | 1.048 | 311/1066 | 77/182 | <0.001 | 0.562 | 388/1248 |
| (31.9) | (30.9) | (0.724-1.516) | (29.1) | (42.3) | (0.407-0.775) | (31.1) | |||
| Imipenem | 99/104 | 694/705 | 0.043 | 3.181 | 727/742 | 66/67 | 1.000 | 0.734 | 793/809 |
| (95.2) | (98.4) | (1.084-9.363) | (98.0) | (98.5) | (0.095-5.647) | (98.0) | |||
| Meropenem | 102/105 | 734/749 | 0.476 | 0.695 | 683/698 | 148/150 | 0.751 | .615 | 831/848 |
| (97.1) | (98.0) | (0.198-2.442) | (97.9) | (98.7) | (0.139-2.720) | (98.0) | |||
| Ceftazidime | 64/127 | 474/1023 | 0.387 | 0.850 | 460/974 | 65/176 | 0.004 | 1.612 | 538/1150 |
| (50.4) | (46.3) | (0.588-1.229) | (48.6) | (36.9) | (1.158-2.245) | (46.8) | |||
| Ceftriaxone | 41/87 | 183/462 | 0.191 | 1.359 | 209/498 | 16/47 | 0.291 | 1.401 | 225/545 |
| (47.1) | (39.6) | (0.857-2.153) | (42.0) | (34.0) | (0.747-2.628) | (41.3) | |||
| Cefotaxime | 37/88 | 180/464 | 0.567 | 0.874 | 201/512 | 16/40 | 0.926 | 0.969 | 217/552 |
| (42.0) | (38.8) | (0.550-1.388) | (39.3) | (40.0) | (0.503-1.870) | (39.3) | |||
| Cefepime | 67/118 | 495/971 | 0.234 | 1.263 | 486/914 | 76/169 | 0.500 | 1.390 | 562/1083 |
| (56.8) | (51.0) | (0.859-1.857) | (53.2) | (45.0) | (0.999-1.932) | (51.9) | |||
| Gentamicin | 80/106 | 357/531 | 0.095 | 0.667 | 399/589 | 38/48 | 0.101 | 0.553 | 437/637 |
| (75.5) | (67.2) | (0.413-1.076) | (67.7) | (79.2) | (0.270-1.133) | (68.6) | |||
| Amikacin | 684/120 | 924/977 | 0.848 | 0.919 | 872/921 | 166/176 | 0.846 | 1.072 | 1038/1097 |
| (95.0) | (94.6) | (0.387-2.184) | (94.7) | (94.3) | (.632-2.159) | (94.6) | |||
| Trimethoprim- sulfamethoxazole | 45/124 | 246/775 | 0.315 | 0.816 | 250/776 | 41/123 | 0.806 | 0.951 | 291/899 |
| (36.3) | (31.7) | (0.549-1.213) | (32.2) | (33.3) | (0.635-1.424) | (32.4) | |||
| Ciprofloxacin | 77/113 | 378/1040 | 0.000 | 0.267 | 399/983 | 56/170 | 0.060 | 1.391 | 455/1153 |
| (68.1) | (36.3) | (0.176-0.404) | (40.6) | (32.9) | (0.986-1.962) | (39.5) | |||
Sensitivity Profile of E. coli in Accordance to Age Group and Source of the Infection in Patients Admitted in three Hospitals in Isfahan, Iran a
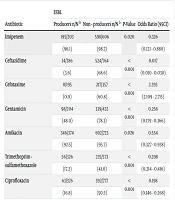
ESBL producers comprised 388 (31.1%) of the isolates. The frequency of ESBL production was more prevalent in nosocomial isolates (42.3%) compared to community-acquired ones (29.1%), and in bloodstream (52.9%) or skin and soft tissue infections (57.1%) compared to other infections. The rate of ESBL production was less common in UTI isolates (25.6%) than in other infections. Susceptibility of E.coli ESBL producers was significantly lower to all examined antibiotics, including Ceftazidime, Cefotaxime, Gentamicin, Amikacin, Trimethoprim-sulfamethoxazole, and Ciprofloxacin, than non-ESBL producing E. coli isolates (Table 2).
| Diagnosis | UTI | Sepsis/Bacteremia | Skin and Soft Tissue Infection | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Antibiotic | n/N a | P- Value | Odds Ratio (95CI) | n/N a | P- Value | Odds Ratio (95CI) | n/N a | P- Value | Odds Ratio (95CI) |
| ESBL + | 230/898 | <0.001 | 0.327 | 100/189 | <0.001 | 2.850 | 56/98 | <0.001 | 3.122 |
| (25.6) | (0.250-0.428) | (52.9) | (2.077-3.912) | (57.1) | (2.051-4.753) | ||||
| Imipenem | 640/653 | 1.000 | 0.965 | 108/111 | 0.472 | 0.683 | 21/21 | 1.000 | 0.974 |
| (98.0) | (0.272-3.429) | (97.3) | (0.192-2.437) | (100) | (0.962-0.985) | ||||
| Meropenem | 561/571 | 0.450 | 1.454 | 135/140 | 0.035 | 0.272 | 86/88 | 0.694 | 0.866 |
| (98.2) | (0.548-3.863) | (96.4) | (0.085-0.868) | (97.7) | (0.195-3.850) | ||||
| Ceftazidime | 383/810 | 0.599 | 1.071 | 93/180 | 0.153 | 1.261 | 30/98 | 0.001 | 0.472 |
| (47.3) | (0.830-1.380) | (51.7) | (0.917-1.734) | (30.6) | (0.302-0.738) | ||||
| Ceftriaxone | 178/434 | 0.800 | 0.947 | 37/79 | 0.278 | 1.303 | 4/18 | 0.142 | 0.396 |
| (41.0) | (0.621-1.444) | (46.8) | (0.807-2.103) | (22.2) | (0.128-1.218) | ||||
| Cefotaxime | 175/445 | 0.989 | 1.003 | 31/79 | 0.989 | 0.997 | 5/13 | 1.000 | 0.964 |
| (39.3) | (0.651-1.545) | (39.2) | (0.612-1.623) | (38.5) | (0.311-2.986) | ||||
| Cefepime | 404/760 | 0.201 | 1.185 | 90/175 | 0.893 | 0.978 | 37/95 | 0.008 | 0.563 |
| (53.2) | (0.913-1.538) | (51.4) | (0.708-1.352) | (38.9) | (0.366-0.866) | ||||
| Gentamicin | 338/508 | 0.026 | 0.602 | 70/92 | 0.094 | 1.543 | 13/16 | 0.414 | 2.013 |
| (66.5) | (0.385-0.943) | (76.1) | (0.925-2.574) | (81.2) | (0.567-7.146) | ||||
| Amikacin | 732/773 | 0.866 | 1.050 | 159/172 | 0.168 | 0.640 | 93/97 | 0.813 | 1.353 |
| (94.7) | (0.594-1.857) | (92.4) | (0.338-1.212) | (95.9) | (0.480-3.817) | ||||
| Trimethoprim- sulfamethoxazole | 204/673 | 0.023 | 0.695 | 51/135 | 0.145 | 1.326 | 19/53 | 0.577 | 1.179 |
| (30.3) | (0.507-0.952) | (37.8) | (0.907-1.938) | (35.8) | (0.661-2.105) | ||||
| Ciprofloxacin | 322/821 | 0.792 | 0.966 | 80/180 | 0.137 | 1.276 | 23/94 | 0.002 | 0.470 |
| (39.2) | (0.744-1.253) | (44.4) | (0.925-1.759) | (24.5) | (0.289-0.764) | ||||
Sensitivity of E. coli Isolates in Accordance to Diagnosis of Infected Patients in Three Hospitals in Isfahan, Iran
The sensitivity of the isolates to examined antibiotics was similar in different infections, except for Gentamicin and Trimethoprim-sulfamethoxazole, which were more effective in UTI isolates, Meropenem, which was less effective in bloodstream infection isolates, and Cefepime and Ciprofloxacin, which were less effective in skin and soft tissue infection isolates compared to other infections (Table 3).
| Antibiotic | ESBL | |||
|---|---|---|---|---|
| Producers n/N b | Non - producers n/N b | P-Value | Odds Ratio (95CI) | |
| Imipenem | 195/203 | 598/606 | 0.020 | 0.326 |
| (96.1) | (98.7) | (0.121 - 0.880) | ||
| Ceftazidime | 14/386 | 524/764 | < 0.001 | 0.017 |
| (3.6) | (68.6) | (0.010 - 0.030) | ||
| Cefotaxime | 0/195 | 217/357 | < 0.001 | 2.393 |
| (0.0) | (60.8) | (2.109 - 2.715) | ||
| Gentamicin | 98/204 | 339/433 | < 0.001 | 0.256 |
| (48.0) | (78.3) | (0.179 - 0.366) | ||
| Amikacin | 346/374 | 692/723 | 0.026 | 0.554 |
| (92.5) | (95.7) | (0.327 - 0.938) | ||
| Trimethoprim - sulfamethoxazole | 56/326 | 235/573 | < 0.001 | 0.298 |
| (17.2) | (41.0) | (0.214 - 0.416) | ||
| Ciprofloxacin | 63/376 | 392/777 | < 0.001 | 0.198 |
| (16.8) | (50.5) | (0.146 - 0.268) | ||
Sensitivity Profile of E. coli in Accordance to Production of ESBL in Infected Patients Admitted in Three Hospitals in Isfahan, Iran a
5. Discussion
Our findings revealed that most E. coli isolates from hospitalized patients in our region had high susceptibility to Imipenem, Meropenem, and Amikacin; moderate sensitivity to Gentamicin and Cefepime; and low susceptibility to Ceftazidime, Ceftriaxone, Ciprofloxacin, Cefotaxime, and Trimethoprim-sulfamethoxazole. In total, 31.1% of the isolates in our study were ESBL producers.
The present study showed that more than 90% of the E. coli isolates were susceptible to Imipenem, Meropenem, and Amikacin. Susceptibility to these antibiotics was high across all age groups, associated infections, and sources of infection acquisition (hospital versus community). As a result, these drugs can be effectively used in the empiric treatment of severe infections across different ages, various infections, and different acquisition sources. Other studies have reported similarly high susceptibility of E. coli to Carbapenems and Amikacin, indicating that these antibiotics can be used in severe infections caused by these bacteria in many parts of the world (10-12).
In our study, resistance to third- and fourth-generation cephalosporins, including Cefotaxime, Ceftriaxone, Ceftazidime, and Cefepime, was high (60.7%, 58.7%, 53.2%, and 48.1%, respectively). These drugs, which were the antibiotics of choice for many years in urinary tract, bloodstream, and wound infections, can now only be advised for non-severe cases or during the de-escalation phase of antibacterial therapy in such infections. High resistance to extended-spectrum cephalosporins was observed in all ages, all infections, and all acquisition sites (community or hospital). The resistance rate to Ceftazidime in hospital-acquired infections (73.1%) was statistically higher than in community-acquired infections (51.4%). However, in practice, a high level of resistance in both groups precludes recommending its use in the empiric treatment of severe infections that E. coli may cause. This high resistance level to third- and fourth-generation cephalosporins necessitates reconsideration in the blind treatment of urinary tract, bloodstream, surgical site, or other infections. Other research in Bangladesh (13), Iraq (11), and Iran (6) has observed similarly high levels of resistance to third- and fourth-generation cephalosporins.
The resistance of isolated E. coli strains in this study to Ciprofloxacin and Trimethoprim-sulfamethoxazole was high (60.5% and 67.6%, respectively). Therefore, these drugs are unsuitable for the empiric treatment of severe infections caused by this bacterium. In previous studies, resistance to these drugs has differed in different regions. The resistance rate to Fluoroquinolones has been reported as 5.5% in North America (14), 27% in Bangladesh (14), 45.5% in Iraq (11), 62.5% in Ethiopia (15), and 82.5% in India (16). On the other hand, resistance to Trimethoprim-sulfamethoxazole was 62.5%, 17.3%, 45.6%, 52.2%, and 82.5% in similar investigations in Ethiopia (15), North America (17), Bangladesh (14), Iraq (11), and India (16), respectively. This difference in the resistance of E. coli can be associated with different sampling sites or geographic variations in the organism's resistance.
In the present study, more than 31% of E. coli strains produced ESBL. These strains were significantly more prevalent in hospital-acquired (42.3%) than community-acquired infections (29.2%) and in bloodstream (52.9%) or skin and soft tissue infections (57.1%) compared to other infections. The rate of ESBL production was less common in UTI isolates (25.6%) compared to other studied infections. The susceptibility of these strains to all examined antibacterials, including Imipenem, Ceftazidime, Cefotaxime, Amikacin, Gentamicin, Ciprofloxacin, and Trimethoprim-sulfamethoxazole, was significantly lower than strains that did not produce this enzyme. In other studies from Iran, a similar prevalence of E. coli strains that produce this enzyme was observed (4-6).
In conclusion, our study showed the high susceptibility of E. coli strains in hospitalized patients to Imipenem, Meropenem, and Amikacin, and a high level of resistance to Ceftazidime, Cefotaxime, Ceftriaxone, Ciprofloxacin, and Trimethoprim-sulfamethoxazole. Clinical guidelines for treating infections where E. coli is a major cause should be revised to include Imipenem, Meropenem, or Amikacin in the empiric therapy of severe infections.