1. Background
The term thalassemia is derived from a Greek word meaning the sea (Mediterranean) in the blood. Thalassemia is caused by the quantitative problems (lack or partial reduction of globin chains). Almost all races in any geographical location face thalassemia disease; however, it is more common in the Mediterranean and tropical regions near the equator in Asia and Africa. The thalassemia is classified based on the affected chain of hemoglobin (1). Regular blood transfusions are the only treatment available for patients with thalassemia that actually improves the symptoms, but it may have some complications and the most important of which is transfusion-transmitted infections such as hepatitis viruses (2).
Acute viral hepatitis is a systemic infection that primarily affects the liver. Viral hepatitis is a disease associated with liver inflammation and necrosis caused by known hepatitis viruses of A, B, C, D, E, G, and T. The hepatitis E (HEV) and A (HAV) viruses are transmitted mainly through oral-fecal route and the other types are transmitted by blood (3, 4). The mean severity of hepatitis E is higher than that of hepatitis A, which its mortality rate is more than 1% (5).
HEV may be observed as epidemic and sporadic cases in the developing countries known as non-A, non-B hepatitis (6). Hepatitis E is a major public health problem in the developing countries of Southeast and Central Asia, the Middle East and North Africa. The disease is reported as epidemic in Mexico and sporadic in industrialized countries (7-10). Serological study of hepatitis E in low prevalence areas is about 27% to 30% and in non-endemic areas about 1% to 2% (11, 12).
A study performed in the city of Sari, Mazandaran province, Iran, indicated that the prevalence of HEV was about 2.3% and increased with age. Another study reported that the prevalence was 7.8% among blood donors. These reports considered Iran as an epidemic area of HEV infection (11-13). Because of the vast area of the country, the prevalence of the disease is different in each region. Since no study was conducted to determine the prevalence of HEV in the Northeast of Iran, the current study aimed at determining the prevalence of the virus among patients with thalassemia compared to the healthy controls.
2. Methods
The current cross sectional study was performed in 2015 in the laboratory of Mashhad blood transfusion organization on peripheral blood samples of 150 patients with beta-thalassemia major and 150 blood donors as the control group sponsored by the research deputy of the school of medicine. The diagnosis of all patients with beta-thalassemia major was performed by a hematologist. Samples with recorded hemolysis and lipemia were excluded from the study.
Personal information of blood donors in the study was extracted from the personal information questionnaires used by the blood transfusion organization. Blood samples were randomly selected according to registration records of all blood donors in all blood transfusion centers in Bushehr province, Iran.
After obtaining informed consent from people participating in the research, a 5-mL peripheral blood sample was taken from each subject. After the separation of plasma/serum, samples were stored at -20°C until testing.
Enzyme-linked immunosorbent assay (ELISA) kit was used to assess HEV susceptibility. All the experiments were conducted according to the manufacturer’s protocol. The enzyme immunoassay microplate reader device capable of measuring the absorbance at 450 nm was used to measure enzyme activities.
The collected data were analyzed by SPSS version 21. Descriptive variables, Chi-square and t tests were used to analyze data. Parametric and non-parametric tests were used for data analysis according to normal or abnormal distribution of data.
3. Results
In the case group, 89 (59.3%) patients were male and 61 (40.7%) female. In the control group, 138 (92%) subjects were male and 12 (8%) female. To investigate the homogeneity of gender in the groups, Chi-square test was used, indicating no significant relationship between the 2 groups (P value = 0.001).
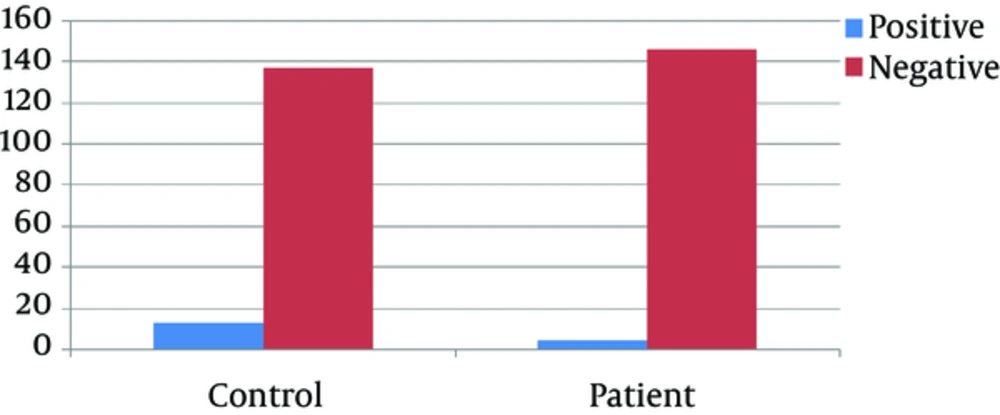
The mean age of the patient and control groups was 26.5 ± 0. 49 years; representing no statistically significant difference between the groups; 13 (8.6%) subjects in the control group and 4 (2.6%) in the patient group had HEV infection.
All people in the control and patient groups were Mashhad residents. Subjects in both groups denoted blood for 2 to 9 times. All results are shown in Figure 1 and Tables 1 and 2.
| Donors | Patients with Thalassemia | P Value | |
|---|---|---|---|
| HCT, %38 | 38 ± 11 | 25.3 ± 5.5 | 0.01 |
| HB, g/dL39 | 11 ± 5 | 7.9 ± 2.5 | 0.018 |
| RBC, 106 µL40 | 3.5 ± 1.5 | 3.5 ± 1.5 | 0.9 |
| PLT, 103µL41 | 238 ± 157 | 290 ± 170 | 0.05 |
aValues are expressed as mean ± SD.
| Control Group | Patients Group | P Value | |||
|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | ||
| O | 36 | 6 | 39 | 5 | 0.001 |
| B | 27 | 4 | 26 | 3 | 0.003 |
| A | 30 | 5 | 31 | 5 | 0.001 |
| AB | 7 | 1 | 4 | 1 | 0.002 |
4. Discussion
Frequent transfusions of blood products may lead to various diseases caused by infectious agents such as hepatitis B and C. There are various reports that HEV infection may be transmitted through frequent transfusions in endemic and non-endemic areas. Therefore, it is important to determine the prevalence of HEV antibodies among high risk patients in each region, due to the high cost of treatment. Epidemiologically, hepatitis E is the leading cause of acute viral hepatitis in the developing countries with poor sanitation and its epidemiological pattern is different in the developed and developing countries.
In the current study, the prevalence of anti-HEV antibodies in patients with thalassemia in Mashhad was 2.6%, which was less than those of Iran and the world. The prevalence in the control group in Mashhad was lower than those of the studies performed on blood donors in Iran (8.6%). The prevalence of hepatitis E in the developing countries was reported 10% to 35% by the seroepidemiological investigations (14). Hepatitis E is the most common cause of acute hepatitis in Asia and the second cause of acute hepatitis in adults followed by hepatitis B in North Africa and the Middle East (15-17). In many studies performed in the Western countries, hepatitis E was investigated in animals, especially pigs and wild boars, and consumption of their products was identified as one of the main causes of infection transmission to humans (18). Although in some of these countries the serological prevalence of HEV is high, the differences of public health status should be considered. In Italy, the outbreak was reported 20.6% (19), in Egypt 17.2% (20), and in Korea 11/2% (21).
Analysis of serum samples collected from blood donors in different industrialized countries including the United States, Britain, France, Germany, Spain, Italy, and Japan identified anti-HEV IgG from 1.1% to 2.2% (22).
A study in Finland showed that HEV should be considered in the differential diagnosis of acute hepatitis. In this region, the prevalence of IgG and IgM was evaluated by ELISA test among 97 patients, which was detected in 29 serum samples (27.6%) (23). In Iran the epidemic disease was reported in the Western areas and Isfahan, and the first documented outbreak occurred in Kermanshah in 1991 (24). A study performed in Hamadan, Iran, in 2005 on blood donors, showed the prevalence of anti-HEV IgG 12.9%.
Given the high prevalence of 5% in the endemic areas, Iran is considered as an endemic region (25). A study performed in Tabriz, Iran, estimated the general prevalence of hepatitis E by 7.8%. The mean age of the cases with positive HEV samples was 40.7 years (26).
In another study performed in Hamadan on 280 blood donors, 249 male (88.9%) and 31 (11.1%) female, anti-HEV IgG was detected in 36 (12.9%) and hepatitis E in 7 subjects (2.5%). Generally, serological prevalence of 12.9% in Hamadan among blood donors, as well as the presence of infection in the cities of Tabriz and Nahavand indicated Iran as an endemic country in terms of HEV infection (24).
In a study by Abolfazl Setoodeh Jahromi et al. on the prevalence of HEV in 110 patients with thalassemia, the results indicated that 10% and 1.8% of patients had anti-HEV IgM and IgG antibodies, respectively. The study also indicated that as the age increased, the need for screening of blood products in terms of HEV is also increased in patients with thalassemia. The study revealed a significant trend of positive serology in the age range of 11 to 20 years; but after 20 years the trend decreased. Seroprevalence of people living in HEV endemic areas and in non-endemic areas decreased significantly with the increase of age. In the current study, subjects were older than 18 years and the frequency was reported based on the age (26).
4.1. Conclusions
The current study results indicated HEV in few patients with major thalassemia.Based on the fact that fecal-oral transmission is the main and proven route of HEV transmission, extra efforts should be made in the field of community health promotion among which the way of disposing waste water properly and sanitarily is a priority. Based on the history of epidemic diseases in several cities of Iran, possible outbreaks should be considered and necessary preventive measures and required trainings should be implemented.
The studies performed so far also demonstrated the possibility of HEV transmission through blood products; therefore, more studies are necessary to prove it. If confirmed, blood products should be screened routinely for HEV by the blood transfusion organization and this should be among the routine tests on donated bloods. One way to prevent the spread of infection is screening the patients with hepatitis and icterus. Therefore, it is recommended that these patients are routinely tested for the contamination with HEV to prevent the transmission of the virus to others, especially pregnant females. It can also make the responsible authorities aware of its high morbidity and mortality, especially in pregnant females, and in addition to primary preventive measures, secondary preventive measures including vaccination should be followed.
It also seems necessary to note the particular importance of the disease during pregnancy and infancy due to a weakened immune system; and based on the fact that pregnant females and infants were not enrolled in the study, it is recommended to consider these groups in future studies to achieve overall and exact prevalence.