1. Background
The investigation of rheumatoid arthritis (RA) cases has revealed that the disease has been a public health concern considering the rising cost trends, its incidence, and disability. Many related researches have been conducted (1). The conclusion of the current investigations showed that RA plays the role of an autoimmune disease in a way that the existence of an antigen or superantigen causes pathological immune reaction. These antigens or superantigens are MHC type II positive cell classification up to CD4 T cells (2). Staphylococcal superantigens (enterotoxins) have been implicated in the pathogenesis of inflammatory diseases. Antigens, which have been generated by Staphylococcus aureus (S. aureus), are classified into the most lethal toxins that rise a toxic shock by destructing a cellular immune response. Chemokines, which are very tiny, have an important role in arthritis (3, 4). Chemoattractant cytokines have an effect in gathering all inflammatory cells near the inflammation (5). Schutyser et al. reported that the monocytic cells react to Gram-positive bacterial infection by CCL18/PARC generation (chemokine ligand 18/pulmonary and activation regulated chemokine) in the synovial cavity (6). Recently, the antagonist activity of this peptide has been identified as the novel domain in superantigens, which is critical for their toxic action (7, 8).
The Staphylococcal enterotoxins (SEs) are superantigens that can disable the T-cell activation and proliferation; their action form is more likely linked to cytokine release action and cell death through apoptosis and some other toxic shock syndrome, which can have a roll of a potentially lethal toxin. Although we could classify SEs superantigen activities, the source of action mode, which causes emesis and diarrhea, has not been yet characterized (9).
It is not reliable to take T-cells proliferation into account as the source of TSST-1, but instead, other host cells receptors should be considered. It was reported that chemokines release is activated by TSST-1 stimulation, like IL-8 and MIP-3a, IL-2, and TNFa (10, 11).
However, PCR (polymerase chain reaction), based on molecular techniques, has led to an increase in diagnosis of bacterial etiologies for clinical specimens with negative bacterial culture (12, 13). Several investigations have also been done to examine the PCR technique for standardization detection of Staphylococcal enterotoxins in body fluids of patients (14-17). In this study, we aimed at detecting Staphylococcal enterotoxin E in synovial fluids of patients with rheumatoid arthritis.
2. Methods
S. aureus has entE gene, the main factor of enterotoxin E, which was isolated from clinical samples and used as positive control (18). The primers pairs were designed based on the reference sequence of the S. aureus enterotoxin type E (entE) gene, GenBank: M21319.1, using online Gene script software. Then, they were analyzed by Primer3 software. In addition, multiple alignments were performed by DNASIS MAX trial version. The final sequence of designed primer pairs was as follows: TCATTGCCCTAACGTTGACA and R CTTACCGTGGACCCTTCAGA.
From April 2011 to August 2013, a total of 100 synovial fluid samples were collected from the patients with RA by rheumatologists and kept at -80°C until used. The criteria for adding or removing a patient were based on ACR 2010 criteria.
The fulfilling of the sampling process by the complete observance of aseptic conditions was based on the microbial standard protocol; 5 to 10 mL synovial fluid was taken from the disinfecting samples using a syringe. Then, 3 to 5 mL was injected into Castaneda medium (BAHARAFSHAN Co, Tehran, Iran) and incubated for 48 hours at 37°C. To perform PCR, DNA was extracted from bacteria as positive control, and then, bacterial standard strain was injected into 5 mL LB and incubated for 24 hours at 37°C. DNA was extracted by cellular sediments and a method named salting-out (14). For DNA extraction from synovial fluid, 100 µL of synovial fluid was picked and injected into DNA- free sterile tube. In the later stage, using a Cinapure DNA extraction kit (CinnaGen Co, thehran Iran), each of the synovial fluid genomes was extracted in isolation. Based on the kit instruction, a volume sized equal to 100 µL (microlitter) was added to the kit’s tube; then, 400 µL of some buffer called lysis was added to suspension and whirlpooled about 20 seconds; afterwards, 300 µL of precipitation was added to the microtube and whirlpooled once more for 5 seconds. At this point, all components were centrifuged (1 minute, 5°C, 13000 × g). Then, we switched to a new microtube and washed the column with 400 µL of buffer number 1 and centrifuged it once more (1 minute, 5°C, 12000 × g). After switching the column itself to a new one, 30 µL of elution buffer was added and centrifuged 5 minutes, 12000 × g). In the last stage, we measured its quality and quantity by NanoDrop (thermo scientific NanoDrop 2000 Spectrophotometer, USA).
To amplify the DNA, a mixture was prepared in 200 µL using a mixture, which would respond to the volume of 25 µL in a sparking rate, which contains 2 µL to assure the amount of 2 µL, 0.3 U from Taq DNA polymerase, buffer size of 2.5 µL of type 10x PCR as well as 0.16 mM of each dNTPs, 2 mM MgCl2 (all indexes were from Cinagen company, Iran ), 10 pmol of initial pair (mingled by Cinagen) and doubled-stilled water to a final amount of 25 µL. All the same amplifications were done in a thermal process by a thermocycler (Bio-Rad, C1000) with deprivation at 95°C, a 3- minute time interval for 35 cycles at 94°C for 30 seconds, and initial heating at 72°C for 5 minutes. The PCR products, which help amplification, have been electrophoresed in a process lasting for 45 minutes, and were then colored with a substance named ethidium bromide or cyber green day in 20 minutes (0.05 mg/mL; Sigma Aldrich). By employing ultraviolet light, the gel was photographed by gel documentation (Bio-Rad universal hood II, USA). In addition, molecular dimensions were in every agarose gel. In the last stage, using an optimization PCR, all samples of synovial fluid were examined separately.
3. Results
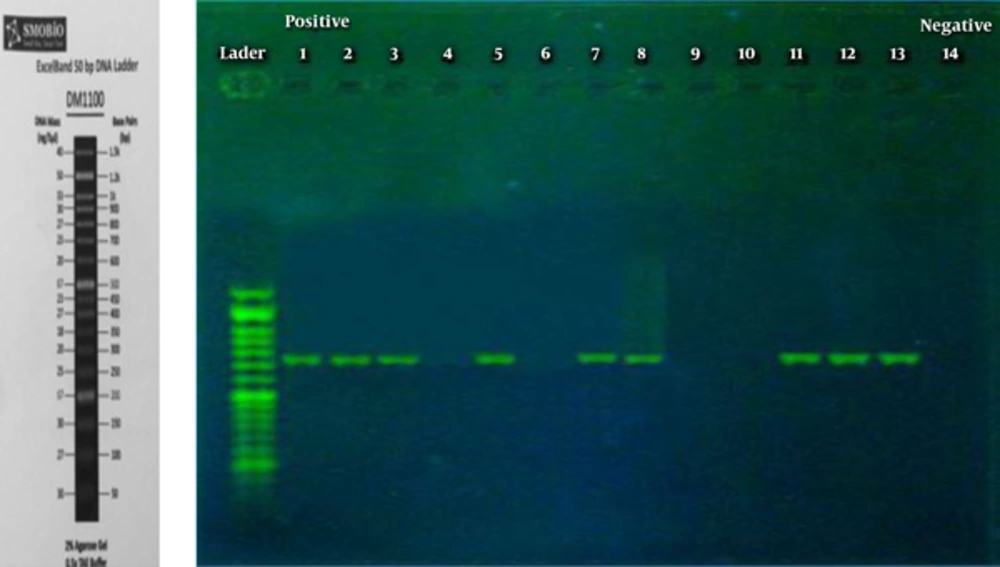
At last, the yielded results indicated that the initial design sequence played a role in amplification of 725 bp amplicons like a product used as PCR. Based on the information related to species genome and the components from master mix, PCR has been improved precisely and employed as a positive aspect of control. Examination of the samples revealed that the PCR method is able to detect entE gene in 25% of patients’ synovial fluid. Figure 1 displayed the results of PCR product, presenting electrophoresed samples related to synovial fluid of patients with RA. The amplicon 725 bp fragments for a number of SF samples were characterized and sequenced.
Figure 1 that the first line is 50 MW marker, lines 1, 2, 3, 7, 8, 11, 12, and 13 are shown as 725 bp fragments (partial entE Gene). Lines 4, 6, 9, and 10 are used as negative control and line 5 as positive control.
4. Discussion
The results of this study revealed the existence of Staphylococcal enterotoxin E gene with the frequency of 25% in synovial fluid samples of patients with RA.
Zahiri Y et al. in their study, examined Staphylococcal enterotoxin E gene in blood samples of patients with rheumatoid arthritis disease. Just 5 bacterial species were isolated, of which one case was found to have Staphylococcus aureus bacterium, based on biochemical test results. The results obtained from molecular examinations revealed the existence of 13.25% of enterotoxin E gene (19). However, in this study, the results were associated with 25% positive Staphylococcal E gene. The differences may be related to the origin of samples. In fact, these reports are among the first published researches results. Zahiri Y et al. reported S.aureus entE in the blood of patients with RA, while this toxin gene was present in the SF of RA patients. However, many studies have been done on the toxigenicity of isolated S. aureus strain from patients (17), foods materials (20), and even on environmental isolated (21). As an example, Rahimi conducted a study to characterize the outbreak linked to enterotoxin producing S. aureus species in uncooked meat and hamburgers in Isfahan province, Iran. Nearly, 223 (60%) of S. aureus were isolated and diagnosed, and among them, 13.5% (equal to 30 strains) were recognized to be enterotoxigenic. Surprisingly, 86% of them (equal to 26 strains) were positive just against one type (14 SEA, 1 SEB, 6 SEC and 5 SED), while the rest were playing a role as positive ones against more than one SEs. None of the isolated ones were positive against SEE (22).
The study results of Arwa and Humodi revealed that the prevalence of enterotoxin A, B, C in the hands of the tested people was 48 strains (29%) of S. aureus. Of the 48 strains, 9 (18.7%) had entrotoxin A gene and 7 (14.5%) had entrotoxin B and C gene (23).
The results of another study revealed that the prevalence of S. aureus toxins was as follows: enterotoxin A: 23.08%; enterotoxin B: 30.77%%; enterotoxin C: 15.38%; enterotoxin D: 15.38%; and enterotoxin C and A 15.38% (24). Superantigens, such as Staphylococcal enterotoxins, play a major role in inflammatory disease. The results of a study (25) raised a question: Where these enterotoxins enter to SF? Thus, this study was performed to answer this question. The results of the present study showed that a high percentage of patients with rheumatoid arthritis had enterotoxin E gene in their synovial fluid. These findings may demonstrate the role of e enterotoxin as a superantigen to induce rheumatoid arthritis. In addition, the PCR technique is highly recommended as a fast method for the detection of enterotoxin E gene in the synovial fluid. However, further studies should also be conducted. Staphylococcal enterotoxin E gene detection as a model may be used for the diagnosis and modification of treatment of rheumatoid arthritis disease.