1. Background
Today, microbial resistance is a significant global health concern. Identified as one of the most critical challenges by the World Health Organization, it is anticipated to result in ten million deaths by 2050 (1-3). Furthermore, the World Health Organization reports that antimicrobial resistance is an inherent phenomenon, wherein microorganisms develop resistance to antibiotics that were previously effective against them (4). According to a 2019 report by the Centers for Disease Control and Prevention (CDC), mortality caused by pathogens resistant to antimicrobial agents exceeded 35,000 individuals annually between 2012 and 2017 (5, 6).
In recent years, there has been an increase in multi-drug resistance among pathogens, particularly Acinetobacter baumannii. This gram-negative, non-fermenting coccobacillus, belonging to the Moraxellaceae family, primarily causes nosocomial infections. These include ventilator-associated and acquired pneumonia, urinary tract infections, meningitis, bacteremia, gastrointestinal infections, and wound skin infections. The presence of Acinetobacter baumannii strains resistant to antibiotics such as carbapenems and colistin, which are considered last-resort treatments, complicates the treatment process within hospital environments (7).
Intrinsic factors responsible for Acinetobacter baumannii's resistance include reduced membrane permeability, increased efflux pump activity, and the production of various beta-lactamase enzymes (8). Noteworthy among these enzymes are carbapenemase and metallo-beta-lactamase, which confer resistance against beta-lactam antibiotics, including penicillins, cephalosporins, carbapenems, and monobactams. These enzymes interfere with bacterial cell wall synthesis, leading to bacterial death. The presence of these beta-lactamase enzymes is commonly observed in Gram-negative bacteria such as Pseudomonas aeruginosa, Klebsiella pneumoniae, Acinetobacter baumannii, and Escherichia coli (9, 10).
Resistance to carbapenems, particularly in Acinetobacter baumannii, is increasing and imposes a significant financial and healthcare burden (11). In 2017, this led the World Health Organization to recognize carbapenem-resistant Acinetobacter baumannii as a high priority on a published list of 12 antibiotic-resistant bacteria posing the greatest threat to modern medicine (12).
2. Objectives
Therefore, this study aimed to identify the antibiotic resistance pattern of Acinetobacter baumannii in Zahedan and investigate carbapenemase and metallo-beta-lactamase-producing isolates using phenotypic methods, which are crucial for diagnosing and treating infections caused by these strains.
3. Methods
3.1. Study Design and Collection of Isolates
In this cross-sectional study, 372 Acinetobacter baumannii strains were isolated from various clinical samples, including blood, urine, cerebrospinal fluid, burn wounds, and other body fluids, from hospitalized patients at Ali Ebne Abitaleb Hospital in Zahedan, Iran, from September 2019 to May 2022. Sampling was carried out using a simple method. To identify the genus Acinetobacter, the samples were first cultured on MacConkey agar and blood agar (Merck, Germany) media. After Gram staining, biochemical tests including carbohydrate fermentation on TSI, motility in SIM, oxidase test, growth at 42 degrees Celsius, and glucose oxidative-fermentative (O-F) test were performed for the initial identification of Acinetobacter baumannii.
3.2. Antibiotic Susceptibility Test
Antibiotic susceptibility testing was carried out using the Disc Diffusion Test (DDT) for all isolates of Acinetobacter baumannii according to CLSI 2022 guidelines. The isolates were categorized as sensitive, resistant, or intermediate to each antibiotic by measuring the respective zone of inhibition and interpreting the results according to CLSI 2022 guidelines (13). The discs used included piperacillin-tazobactam (100+10 µg), ceftazidime (30 µg), cefepime (30 µg), imipenem (10 µg), meropenem (10 µg), amikacin (30 µg), gentamicin (10 µg), tobramycin (10 µg), ciprofloxacin (5 µg), minocycline (100 µg), and ampicillin-sulbactam (20 µg) (MAST, UK). Additionally, the E. coli strain ATCC 2922 was used as quality control.
3.3. e-Test Method
e-test strips (Liofilichem, Italy) were used to determine the susceptibility of colistin, and the results were interpreted according to CLSI 2022 guidelines (13).
3.4. The Combined Disk Diffusion Test (CDDT)
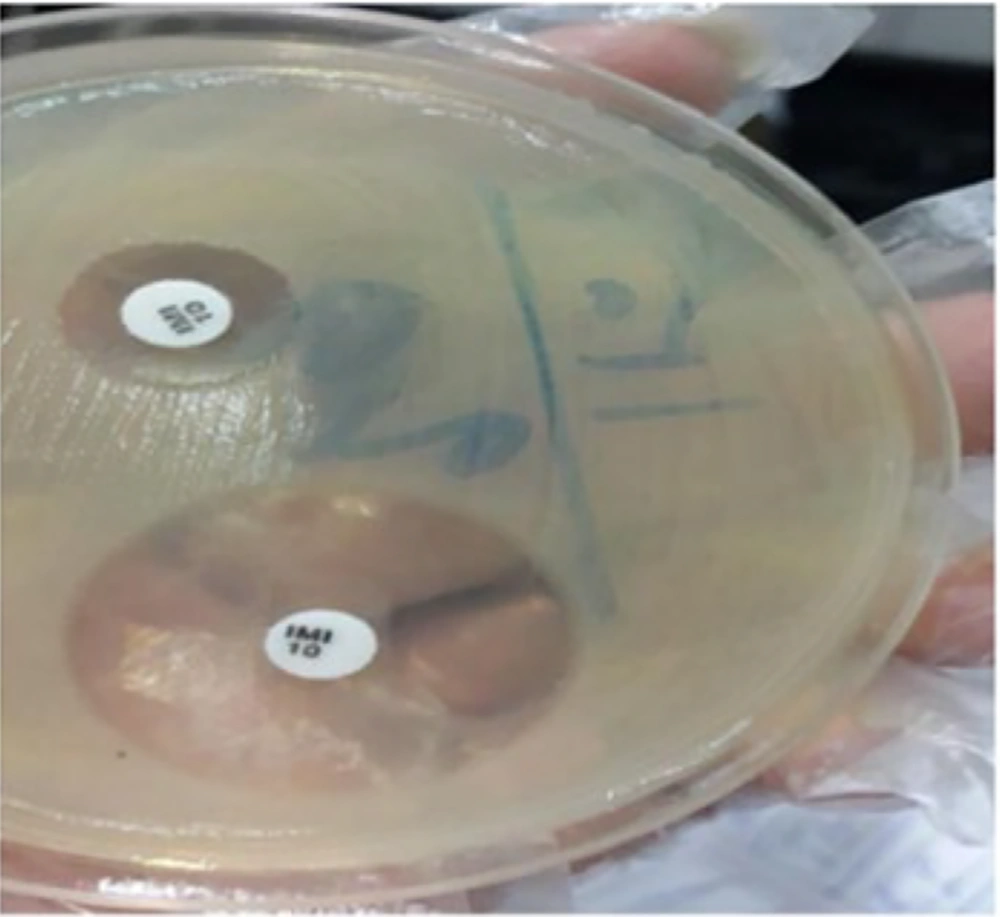
The production of metallo-beta-lactamases in Acinetobacter baumannii strains was assessed using the combined disk diffusion method. Initially, the desired bacteria were cultured on Mueller-Hinton agar medium equivalent to a 0.5 McFarland suspension for 18 to 24 hours, and then cultured on another Mueller-Hinton agar medium. Two discs were placed on the culture medium: One containing 10 µg of imipenem and the other containing imipenem and EDTA, with a minimum distance of 2.5 cm between them. The plates were then incubated at 37 degrees Celsius for 18 to 24 hours. After incubation, an increase in the diameter of the zone of growth inhibition (greater than or equal to 7 millimeters) around the imipenem+EDTA disc alone was considered indicative of metallo-beta-lactamase production (Figure 1).
3.5. The Modified Hodge Test (MHT)
This test was used to detect the production of carbapenemase in Acinetobacter baumannii strains. According to the CLSI guidelines for the Hodge test, a 0.5 McFarland standard suspension of E. coli ATCC 25922 (a carbapenem-sensitive E. coli strain) was prepared and diluted with sterile saline at a 1: 10 ratio. The diluted E. coli suspension was uniformly inoculated on a Mueller Hinton Agar (MHA) medium plate, and allowed to dry for about 5 minutes. A disc containing 10 µg of ertapenem was placed at the center of the inoculated MHA plate. Using a sterile swab, 3 - 5 colonies of freshly cultured Acinetobacter baumannii (test organisms) and Klebsiella pneumoniae ATCC BAA-1705 (positive control) were streaked in a straight line from the edge of the disc to the edge of the plate. The plate was then incubated at 35 (± 2) °C for 16 - 24 hours. Following incubation, enhanced growth of the E. coli and the formation of clover leaf-like indentations at the intersection of the streak of the test organism (and controls) and the zone of inhibition of the E. coli were observed.
3.6. Statistical Analysis
Statistical analysis was performed using the Student t-test and Tukey HSD with SPSS version 23.
4. Results
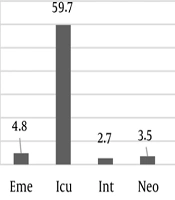
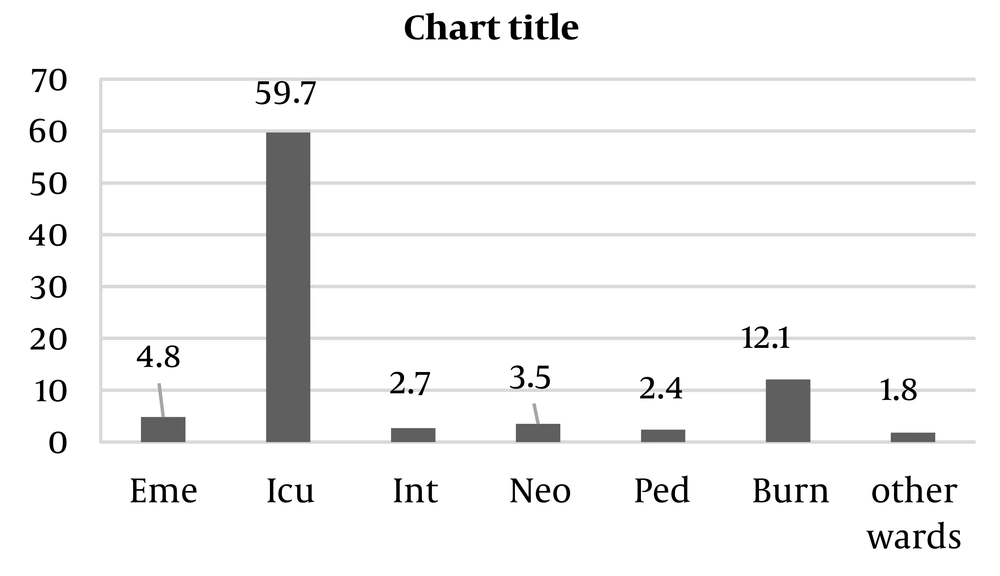
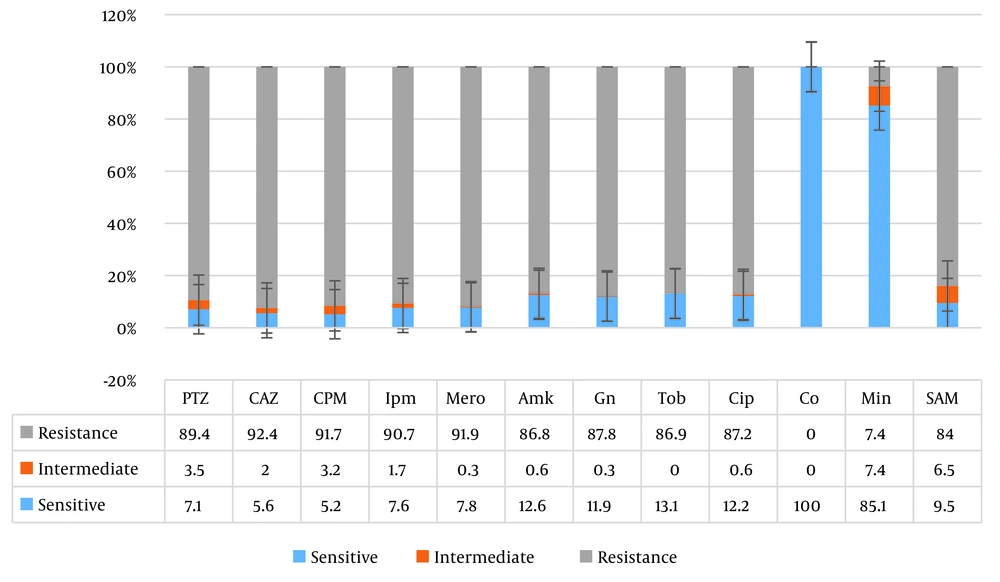
Among the 372 isolates of Acinetobacter baumannii, 206 (55.4%) were collected from male patients and 166 (44.6%) from female patients. The most frequent clinical samples were blood (39.5%), endotracheal tube (ETT) (34.4%), and wound (20.7%). As shown in Figure 2, most samples were collected from patients hospitalized in the ICU ward (59.7%), followed by the burn ward (12.1%) and the emergency ward (4.8%). The antibiotic susceptibility test showed the highest resistance to ceftazidime (92.4%), cefepime (91.7%), and meropenem (91.4%). Resistance to minocycline (7.4%) was the least detected among the isolates. According to the MIC test, all isolates were sensitive to colistin (Figure 3).
4.1. The Results of the Combined Disk Diffusion Test (CDTT)
Out of 372 strains of Acinetobacter baumannii, 352 strains showed a ≥ 7 mm increase in the zone diameter with the Imipenem + EDTA disk compared to the Imipenem disk alone. This result indicates that 94.6% of the isolates produced the metallo-beta-lactamase enzyme.
4.2. Results of the Modified Hodge Test (MHT)
According to the results of this test, 215 strains (57.7%) of Acinetobacter baumannii were strongly positive, 135 strains (36.1%) were weakly positive, and 23 strains (6.2%) were completely negative (Figure 4).
5. Discussion
In this study, antibiotic resistance and the production of carbapenemase and metallo-beta-lactamase in Acinetobacter baumannii strains isolated from clinical samples in Zahedan were investigated using phenotypic methods. Although carbapenems have been the drug of choice for treating infections caused by MDR strains, their widespread use in recent years has led to an increase in carbapenem-resistant strains (14). Recent research has explored the use of antibiotics such as amikacin, polymyxin, tigecycline, minocycline, tobramycin, piperacillin/tazobactam, carbapenems (imipenem, meropenem, doripenem), and colistin to treat infections caused by Acinetobacter baumannii (15).
Additionally, some studies showed that resistance to carbapenems was 90 to 100% during 2016 to 2017 in Iran (16). In other countries, the resistance rate has been within the range of 85 to 98% (17). The results of this study showed that the highest sensitivity was to minocycline (85.1%), and the highest resistance was to ceftazidime (92.4%). Therefore, prescribing cephalosporin antibiotics would not be suitable for the therapeutic management of these infectious conditions.
Salehnia and Nojomi in Guilan demonstrated that 65% of Acinetobacter baumannii strains were resistant to gentamicin and 60% of isolates were resistant to piperacillin/tazobactam. These results differ from those of the present study (18). Pachon-Ibanez et al. in Spain showed a 78% resistance to imipenem during their study (19). Additionally, Gao et al. recorded a 93.75% resistance to imipenem in their study (20).
Initial studies in the 1970s showed that minocycline was effective for treating infections caused by Acinetobacter baumannii. However, at that time, Acinetobacter species were sensitive to most common antibiotics available, so minocycline was not used. The prevalence of MDR strains led to a reassessment of old but effective drugs for treatment, such as minocycline, resulting in the CLSI reclassifying this antibiotic as a group B effective antibiotic for treating Acinetobacter. The study by Adibhesami et al., conducted from 2011 to 2013 in Tehran, indicates that minocycline is still an effective treatment option for Acinetobacter infections (21). Denys et al. in the United States reported that 98 to 100% of Acinetobacter baumannii strains isolated from 2005 to 2011 were sensitive to minocycline (22).
Furthermore, in the present study, the highest number of strains were isolated from the ICU ward. Therefore, it is suggested to design some preventive strategies to reduce nosocomial infections caused by Acinetobacter baumannii in the ICU.
5.1. Conclusions
According to the results of the present study, all strains were sensitive to colistin, and the lowest resistance was observed with minocycline (4.7%). Therefore, considering the limitations of colistin (being expensive) and minocycline (not recommended for pregnant women and children), the use of these drugs, or a combination of them, is recommended for treatment. Additionally, it is crucial to supervise the use of antibiotics in hospitals, especially in ICU wards.