1. Background
The novel virus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), first emerged in Wuhan, China, in December 2019. It rapidly spread worldwide, leading to a global pandemic and a significant health crisis (1). Syndrome coronavirus 2 is an RNA virus belonging to the Betacoronavirus genus and is primarily transmitted through inhaling respiratory droplets from infected individuals or by direct contact with contaminated surfaces and objects (2). By September 23, 2023, at least 6.9 million deaths had been attributed to this disease (3). Patients with chronic conditions such as heart disease, diabetes, chronic kidney disease, hypertension, cancer, and pregnancy have experienced worse outcomes when infected with COVID-19 (4, 5).
Studies suggest that viral infections like hepatitis B virus (HBV) or human immunodeficiency virus (HIV) can also lead to poor outcomes in COVID-19 patients due to immune deficiencies or complications (6, 7). However, recent studies have produced conflicting results on this topic (8). Despite advancements in vaccination, diagnosis, and treatment, HBV remains a significant public health issue with a high mortality rate. The World Health Organization (WHO) reported that nearly 1.5 million people were infected with chronic hepatitis B (CHB) in 2019, with approximately 820,000 deaths attributed to HBV that year (9). Hepatitis B virus spreads through contact with infected blood or body fluids, particularly during childbirth, through injections, or via sexual transmission. It can lead to acute and chronic hepatitis infections, fibrosis, cirrhosis, and hepatocellular carcinoma (HCC) (10).
Studies have shown that the use of immunodeficiency medications or interactions between viruses can affect the suppression of infectious agents. For instance, co-infection with hepatitis B and C (HCV) has been observed, where HCV can inhibit the proliferation of hepatitis B. Consequently, Direct-Acting Antivirals (DAAs) used to achieve a sustained virologic response (SVR) for HCV may cause HBV reactivation if not accompanied by nucleoside/nucleotide analogue (NA) HBV therapy (11).
Research into the impact of COVID-19 on patients with CHB has yielded varying results, with most studies indicating a poor prognosis for these patients. Notably, many of these studies have focused on patients from China, where HBV prevalence is high. In a large cohort from Wuhan, China, it was found that 2.1% of patients had underlying CHB, determined solely by the presence of HBsAg, with no information on antiviral therapy (11). Conversely, Anugwom et al. reported HBV rates in COVID-19 patients ranging from 0 to 1.3%, which is lower than the incidence in the general population of similar ages (12). This discrepancy raises questions about the lower prevalence of HBV infection among hospitalized COVID-19 patients.
2. Objectives
The present study investigates the impact of HBV infection on COVID-19 among non-complicated hepatitis B patients.
3. Methods
3.1. Study Design and Participant's Selection
This cross-sectional study focused on patients with CHB diagnosed with COVID-19, as well as healthy COVID-19 patients, during the third wave of the pandemic. All participants were referred to the infectious disease clinic at Sayyad Shirazi Hospital in Gorgan, Iran, on an outpatient basis. Following examination by an infectious disease specialist, their conditions were evaluated. Inclusion criteria were as follows: Patients over 18 years old, CHB defined as HBsAg positive for at least six months (13), with or without antiviral treatment with tenofovir (TDF) at the time of COVID-19 diagnosis; no history or presence of hepatitis-related complications; and confirmation of SARS-CoV-2 infection using the fluorescent reverse transcription quantitative real-time polymerase chain reaction (RT-qPCR) method. Exclusion criteria included the use of more than one antiviral drug, co-infection with HIV, HCV, or HDV, or the presence of other underlying diseases. An HBsAg level of less than 1000 IU/mL and HBV DNA level of less than 2000 IU/mL indicates "inactive carriers," with a positive predictive value (PPV) of 86% (14). All participants received at least one dose of the COVID-19 vaccine. The study comprised 300 patients, of whom 150 had a history of hepatitis B infection (50 were carriers, and 100 were treated with TDF), forming the hepatitis group with COVID-19 infection. The remaining 150 were healthy COVID-19-infected patients without underlying diseases.
3.2. Data Collection
A patient record system was used to collect patients' demographic data and clinical characteristics. Information on clinical characteristics, management, hospital stay, and outcomes related to COVID-19 was gathered based on medical history. Liver fibrosis was assessed using the results from liver elastography or the most recent liver biopsy.
The use of the PCR method for diagnosing infectious diseases has increased due to its high sensitivity and specificity (15). Therefore, the COVID-19 RT-qPCR was employed in this study to confirm the diagnosis of COVID-19 (16).
3.3. Statistical Analysis
Data were analyzed using Statistical Package for the Social Sciences version 25.0 (SPSS Inc., Chicago, IL), and a P-value of less than 0.05 was considered statistically significant. The outcomes are expressed as mean ± standard deviation or standard error. Chi-square tests and Student's t-tests were used to examine and compare the relationships between variables.
3.4. Ethics Approval
This study was conducted in accordance with the principles of the declaration of Helsinki. Approval was granted by the Golestan University of Medical Sciences Ethics Committee. Approved ethics number: IR.GOUMS.REC.1400.077.
4. Results
All 300 patients included in the study were referred to an outpatient infectious disease clinic, where their status was evaluated after examination by an infectious disease specialist. The study comprised 150 patients with a history of hepatitis B infection (50 carriers and 100 treated with TDF) (Table 1) with COVID-19, forming the hepatitis group, and 150 healthy COVID-19-infected patients without a history of underlying disease, forming the control group.
| Variables | CHB Patients | P-Value | |
|---|---|---|---|
| Active Patients; (n = 100) | Carrier Patients; (n = 50) | ||
| Age (y) | 41.78 ± 12 | 38.82 ± 8.82 | 0.01 |
| Gender | 0.05 | ||
| Female | 60 (60) | 38 (76) | |
| Male | 40 (40) | 12 (24) | |
| Ethnic | 0.08 | ||
| Fars | 59 (59) | 22 (44) | |
| Sistani | 28 (28) | 14 (28) | |
| Turkmen | 12 (12) | 11 (22) | |
| Other | 1 (1) | 3 (6) | |
| Symptoms | 0.5 | ||
| Mild | 60 (60) | 33 (66) | |
| Moderate | 31 (31) | 15 (30) | |
| Severe | 9 (9) | 2 (4) | |
| Hospitalization | 0.1 | ||
| Yes | 5 (5) | 0 (0) | |
| No | 95 (95) | 50 (100) | |
| Ward | N.A. | ||
| ICU | 4 (80) | 0 (0) | |
| Non-ICU | 1 (20) | 0 (0) | |
| Outcome | N.A. | ||
| Mortality | 0 (0) | 0 (0) | |
| Survival | 100 (100) | 50 (100) | |
Abbreviations: CHB, chronic hepatitis B; ICU, intensive care unit; N.A., not applicable
a Values are expressed as Mean ± SD or No. (%).
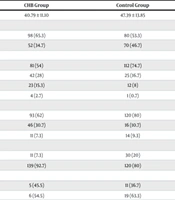
Regarding gender distribution, there were 178 female patients (59.4%) and 122 male patients (40.6%). In the HBV group, there were 98 females (65.3%) and 52 males (34.7%), while in the control group, there were 80 females (53.3%) and 70 males (46.7%). The statistical significance of COVID-19 infection differed between female and male patients in the HBV group (P-value = 0.03).
The mean age of the patients was 44.09 ± 12.96 years. The mean age of HBV patients was 40.79 ± 11.10 years, compared to 47.39 ± 13.85 years in the control group. Most participants were under 44 years old. A statistically significant association was observed between COVID-19 infection and age in the HBV patient group (P-value < 0.001).
The majority of patients were Fars (193 patients, 63.7%), followed by Sistani (67 patients, 22.1%), Turkmen (35 patients, 11.6%), and other ethnicities (5 patients, 1.7%). COVID-19 infection among HBV patients of Fars ethnicity was statistically significant (P-value < 0.001).
None of the patients presented liver fibrosis or other complications, such as ascites, esophageal varices, or HCC. There were no significant differences between groups in HBeAg status, detectable HBV DNA, or treatment duration in the hepatitis group.
According to Table 2, most patients in both groups experienced mild, moderate, or severe symptoms. Severe symptoms were more common in the control group (9.3% vs. 7.3%), and the hospitalization rate was higher in the control group (20%). Both the hospitalization rate and symptom severity were statistically significant (P-value < 0.001).
| Variables | CHB Group | Control Group | P-Value |
|---|---|---|---|
| Age | 40.79 ± 11.10 | 47.39 ± 13.85 | < 0.001 |
| Gender | 0.03 | ||
| Female | 98 (65.3) | 80 (53.3) | |
| Male | 52 (34.7) | 70 (46.7) | |
| Ethnic | < 0.001 | ||
| Fars | 81 (54) | 112 (74.7) | |
| Sistani | 42 (28) | 25 (16.7) | |
| Turkmen | 23 (15.3) | 12 (8) | |
| Other | 4 (2.7) | 1 (0.7) | |
| Symptoms | < 0.001 | ||
| Mild | 93 (62) | 120 (80) | |
| Moderate | 46 (30.7) | 16 (10.7) | |
| Severe | 11 (7.3) | 14 (9.3) | |
| Hospitalization | < 0.001 | ||
| Yes | 11 (7.3) | 30 (20) | |
| No | 139 (92.7) | 120 (80) | |
| Ward | 0.60 | ||
| ICU | 5 (45.5) | 11 (36.7) | |
| Non-ICU | 6 (54.5) | 19 (63.3) | |
| Outcome | 0.15 | ||
| Mortality | 0 (0) | 2 (1.3) | |
| Survival | 150 (100) | 148 (98.7) |
Abbreviations: CHB, chronic hepatitis B; ICU, intensive care unit.
a Values are expressed as Mean ± SD or No. (%).
Additionally, hospitalization rates for moderate and severe disease between the two groups were statistically significant (P-value < 0.001).
Among the 150 patients with hepatitis B, the relationship between TDF consumption and hospitalization or the degree of infection was not statistically significant (P-value = 0.51, P-value = 0.26).
Most patients did not require hospitalization and were treated with outpatient medicine, with a mean treatment duration of 14 days. However, 11 HBV patients and 30 patients in the control group were hospitalized (P-value < 0.001). Among the admitted patients, 11 from the control group and five from the hepatitis group were admitted to the ICU (P-value = 0.6). All patients in the hepatitis group survived, while two patients in the control group died (P-value = 0.15).
5. Discussion
The impact of COVID-19 on HBV patients is not well understood. Hepatitis B virus remains a significant public health issue, with approximately 257 million people suffering from CHB and nearly 900,000 deaths each year worldwide due to complications such as cirrhosis and HCC (17). The effect of HBV on the progression or suppression of other infections is still unclear, and it remains uncertain whether COVID-19 infection in hepatitis patients leads to more severe or milder symptoms and outcomes.
In our cross-sectional study, we investigated HBV patients regarding infection, disease severity, hospitalization needs, and disease outcomes, comparing them with COVID-19 patients without underlying conditions. Our study revealed that hepatitis B patients with COVID-19 experienced less severe infections, lower hospitalization rates, and no mortality compared to the control group.
Studies on the incidence of COVID-19 in hepatitis patients have produced varied results. Most of these studies have been conducted in China, where the prevalence of HBV is around 7% (18, 19). For example, a study in Wuhan, China, involving 1,099 hospitalized patients, found that 2.1% had HBV infection (18). Conversely, another study in China by Chen et al., 2020, involving 123 COVID-19 patients, reported that 12.2% had HBV infection, with these patients experiencing higher rates of severe COVID-19 and increased mortality (20). In contrast, a large study in the United States with 5,700 hospitalized COVID-19 patients found only 0.1% had CHB (21). Additionally, a Korean cohort study found a significant association between underlying CHB and a low SARS-CoV-2 test positivity rate (22). According to this study, the rates of HBV among COVID-19 patients ranged from 0 - 1.3%, which is lower than the incidence in the general population of similar ages (12).
It appears that CHB may influence the nature, infectivity, and function of COVID-19 due to its effects on the immune system, interactions with other viruses, and the role of antiviral treatment. These aspects are discussed in the following sections.
Tenofovir disoproxil fumarate (TDF), known for its efficacy as a reverse transcriptase enzyme antagonist in HIV and HBV, has also been explored for its potential antiviral effects on other viruses, including coronaviruses like SARS-CoV-2. Research indicates that TDF exhibits broad antiviral properties by hindering viral replication through various mechanisms, such as inhibiting viral enzymes and disrupting viral nucleic acid synthesis. While TDF may not directly target the RNA-dependent RNA polymerase (RDRP) of coronaviruses, it could potentially affect other critical processes in the virus's life cycle. Additionally, TDF has been observed to have immunomodulatory effects, reducing the production of interleukin (IL)-8 and IL-10, which are associated with COVID-19 severity. In contrast, other medications, such as tenofovir alafenamide (TAF), have shown less impact on these factors (23-25).
Previous studies have demonstrated that SARS-CoV-2 can trigger the production of various cytokines, including IL-6 and TNF-α, some of which can inhibit HBV infection (26, 27). In a study by He et al., 15 out of 571 COVID-19 patients (2.6%) had HBV infection, and these patients were observed to have a lower risk of severe events (28). However, some studies have shown conflicting results. For example, Chen et al., 2020, reported that among 326 COVID-19 patients, 20 (6.1%) had HBV co-infection, with no significant differences in discharge rates or length of hospitalization (29). Furthermore, Mateos-Muñoz et al., 2023, suggested that TDF may have protective effects for patients with HBV and COVID-19, as these patients had lower rates of ICU admission, need for ventilatory support, hospitalization length, and mortality compared to those on entecavir (ETV) (30). These findings align with previous studies indicating lower COVID-19 severity in HIV patients on antiviral regimens containing TDF (31). A large cohort study in Spain found that the incidence of COVID-19 in CHB patients treated with TDF was decreased, indirectly suggesting a positive effect of TDF on resistance to SARS-CoV-2 (32). Similar clinical observations have been documented in patients with HIV and other viral diseases, with a study showing that HIV patients on combination drugs, including TDF and emtricitabine (TDF/FTC), had a lower rate of SARS-CoV-2 diagnosis and better COVID-19 outcomes (33). However, in our study, there was no significant relation between TDF use and hospitalization or disease severity among hepatitis B patients, which may be due to the small sample size of the CHB patient group.
The impact of host immune status on SARS-CoV-2 infection is another important aspect that requires investigation. Long-term HBV infection leads to various immunomodulatory effects, including a weakened or absent virus-specific T-cell response. This condition, known as "exhaustion," is characterized by reduced cytotoxic activity, impaired cytokine production, and persistent expression of multiple inhibitory receptors. It may also influence the body's response to other viruses, potentially reducing susceptibility to SARS-CoV-2 infection (34, 35). Recent research suggests that T-cell exhaustion may affect responses to viruses like SARS-CoV-2, potentially resulting in less severe disease and a reduced cytokine storm in COVID-19 patients. This raises the question of whether this observation is an epidemiological coincidence or a genuine result of immune dysregulation and could provide additional avenues for preventing and treating COVID-19 (34-36). In our study, hepatitis B patients had lower hospitalization rates and lower rates of moderate and severe disease compared to the control group, with statistical significance (P-value < 0.001).
In most studies on hepatitis patients with COVID-19, those who died generally had liver dysfunction or complications related to hepatitis. Our study included hepatitis B patients without complications to eliminate the potential impact of hepatitis B-related complications on patient mortality. None of these patients died in our study. Consistent with our findings, a large international study involving over 150 case reports of COVID-19 in patients with chronic liver disease indicated higher mortality in those with chronic liver disease and cirrhosis. Hepatic decompensation occurred in 36.9% of these patients and was strongly linked to an increased mortality risk, even in the absence of respiratory symptoms (37). Another study of 50 cirrhotic patients with COVID-19 reported a higher mortality rate, suggesting that COVID-19 may exacerbate liver function and increase mortality in cirrhotic patients. The severity of HBV cirrhosis may contribute to the higher mortality rate in these patients (38). In our study, all HBV patients survived, while two patients (1.3%) in the control group died, which was not statistically significant (P-value = 0.15).
A strength of our study is that prior research did not have adequate data to ascertain the stage of HBV infection or the proportion of CHB patients who were carriers or receiving antiviral treatment. All cases in our study were from a single hospital in one province, which minimized selection bias.
A limitation of our study is the relatively small number of COVID-19 cases with concurrent HBV infection. Larger studies are needed to confirm our findings. The incidence of hepatitis B is much higher than other types of hepatitis. Our research focused specifically on hepatitis B, and the impact of different types of hepatitis on COVID-19 warrants further exploration.
5.1. Conclusions
Recent studies have shown that patients with immunosuppressive conditions, such as CHB and HIV, have a lower risk of developing severe COVID-19 and death. Effective standardized antiviral treatment protocols for COVID-19 patients improve outcomes. Our results suggest that hepatitis B in COVID-19 patients has a protective effect compared to healthy COVID-19 patients, as evidenced by lower duration and rates of hospitalization, intensive care admission, severe infection, and death in CHB patients compared to those without underlying disease. Clinical interventions for COVID-19 patients with CHB are recommended, particularly concerning risk factors associated with disease severity and outcomes, to protect against COVID-19 infection and develop tailored treatment regimens for this population.