1. Background
Pseudomonas aeruginosa is one of the most antibiotic-resistant bacteria which plays an important role in the inception of nosocomial infections all over the world (1). In most parts of the world, resistance of P. aeruginosa against beta-lactam compounds is increasing (2). Recently by using carbapenems, infections due to resistant species are controlled better, however in the last several decades, resistance of P. aeruginosa has been reported against these compounds (3). Although different reasons can be considered for this resistance (such as generation of chromosomal Amp-c, loss of purine D2 or efflux pumps), the most important reason is the generation of carbapenem-hydrolyzing enzymes (4). Metallo-beta-lactamase-producing P. aeruginosa species were reported for the first time in Japan in 1991 (5). However, these species are reported all over the world as well as Iran in which isolation of multidrug-resistant P.aeruginosa species has reached a critical point (5). Metallo-beta-lactamases such as VIM, IMP and SPM which constitute Ambler class B, cause hydrolysis of a variety of beta-lactams except for monobactam (aztreonam) (6). Among beta-lactamase, MBLs are unique in requiring the presence of zinc ion in the active site of the enzyme; although inhibited by chelating agents such as ethylene diamine tetra acetic acid (EDTA), sodium mercaptoacetic acid (SMA), and dipicolinic acid, beta-lactamase inhibitors such as clavulanic acid, sulbactam and tazobactam have no effects (7). Based on epidemiologic studies conducted all over the world, it has been proved that the prevalence rate of genes coding metallo-beta-lactamase in P. aeruginosa lineages are different in various geographic zones, even in various hospitals of a country (8). Therefore, according to the clinical importance of microorganisms producing metallo-beta-lactamase, it is necessary to identify and control them in hospitals for therapeutic purposes (8).
2. Objectives
The current study aimed to investigate the presence and abundance of metallo-beta-lactamase genes in P. aeruginosa species isolated from patients hospitalized in Zahedan hospitals and also to determine the pattern of their antibiotic resistance.
3. Materials and Methods
Samples included 191 isolates of P. aeruginosa collected from Zahedan hospitals in nine months identified by microbiological and biochemical methods. Antibiotic resistance was tested by disk diffusion method with ceftizoxime (ZOX), gentamicin (GM), ciprofloxacin (CP), ceftazidime (CAZ), piperacillin (PLP), tazobactam-piperacillin (TZP), ceftriaxone (CRO), cefotaxime (CTX) and imipenem (IPM) (Mast Co., Britain), according to CLSI standards. Minimum Inhibitory Concentration (MIC) of imipenem resistant isolates was measured by E-test method, according to CLSI protocol. To investigate the antibiotic resistant pattern, P. aeruginosa ATCC7853 was used as the quality control strain. In order to identify the isolates producing MBL, E-test tapes were used (AB Biodisk, Solna Sweden) according to the manufacturer’s instructions. These tapes have two parts; in one part (IP), a gradient of different imipenem ratios (4-256 µg/ml), and in the other one (IPI), a gradient of different imipenem ratios plus a constant concentration of EDTA (1-64µg/ml) are located (7). To interpret E-test tapes, it should be considered that if MIC of IP ratio to IPI is equal or greater than eight, then metallo-beta-lactamase (MBL) enzyme is produced (7). Boiling method was applied for DNA extraction. First, three to five colonies were picked from the fresh culture medium and then to prepare a suspension, 200 mL of distilled water was boiled at -100˚C for 10 minutes and centrifuged at 12000 rpm for 10 minutes. The provided suspension containing DNA was transferred to the new Ependroff tubes to amplify Polymerase Chain Reaction (PCR) in order to assure the presence of metallo-beta-lactamase genes bla-VIM-1, bla-IMP-1, and bla-SPM-1 in imipenem resistant isolates. The specific primers of these genes, which were used to amplify PCR, have been presented in Table 1.
| Primer | Sequence (5′ to 3′) |
|---|---|
| VIM-1 | AGTGGTGAGTATCCGACAG |
| ATGAAAGTGCGTGGAGAC | |
| IMP-1 | ACCGCAGCAGAGTCTTTGCC |
| ACAACCAGTTTTGCCTTACC | |
| SPM-1 | GCGTTTTGTTTGTTGCTC |
| TTG GGGATGTGAGACTAC |
One microliter of the extracted DNA was added to PCR Master Mix with the final volume of 25µl (each vial contained 1.5 Mm of MgCl2, 0.24 Mm of dNTPs, 5 Pm of each prime,r and one unit of Taq polymerase enzyme). PCR test was controlled by P. aeruginosa isolate producing SPM-1 and another isolate producing VIM-1 and IPM-1. In order to control the molecular weight of the amplified product, 100 base pare ladder was applied, the samples were electrophoresed in 1% agarose gel, stained with ethidium bromide and the final results were observed by gel documentation instrument with ultraviolet (UV) light.
4. Results
Among the 191 isolated P. aeruginosa species, the maximum and minimum isolations belonged to ICU (43 isolates) and CCU (one isolate), respectively. Since species were isolated from different clinical samples, most of them (71 isolates) belonged to mid-stream urine and just one isolate belonged to biopsy samples. To determine the antibiotic resistance pattern of P. aeruginosa, Kirby-Bauer method was applied to 191 isolates, collected from different parts of Zahedan hospitals, against piperacillin, cefotaxime, ceftizoxime, ceftazidime, imipenem, tazobactam-piperacillin (TZP) and ciprofloxacin. The results are shown in Table 2.
| Antibiotic | Ceftizoxim | Ceftazidim | Ciprofloxacin | Pipracilin | Imipenem |
|---|---|---|---|---|---|
| Percentage of resistance | 41.88 | 62.8 | 47.1 | 35.6 | 5.7 |
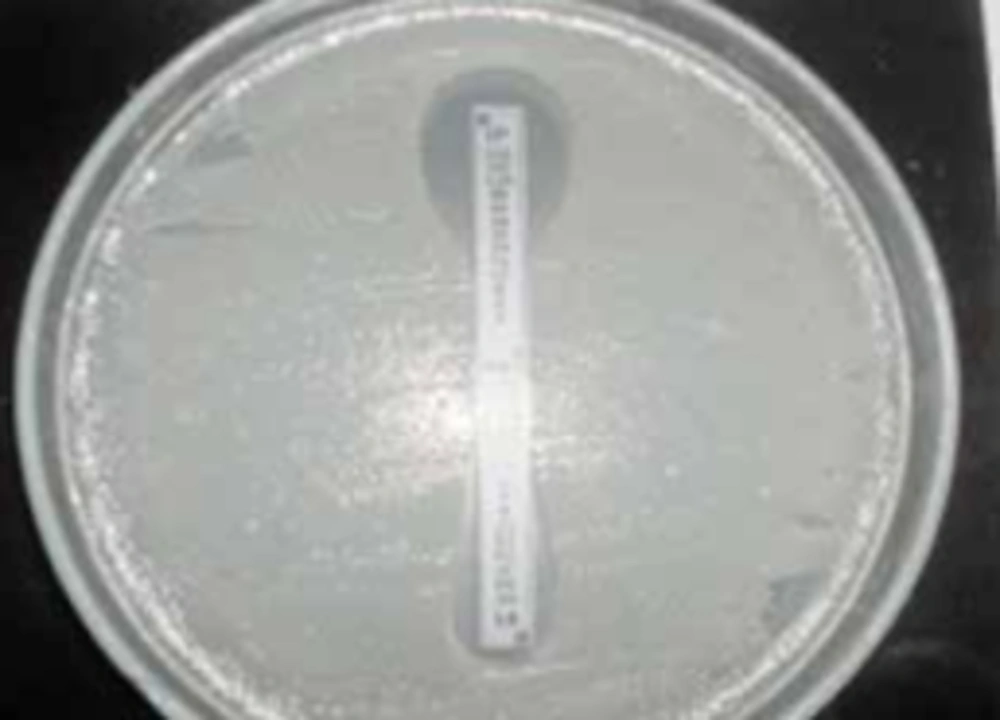
The maximum and minimum resistance belonged to ceftriaxone (67.5%) and imipenem (5.7%), respectively. Eleven out of 191 isolates showed resistance to imipenem. E-test was employed to evaluate these resistant isolates. E-test MBL is a phenotypic method applied to identify metallo-beta-lactamase isolates, and nine out of 11 resistant isolates, were recognized as producer of the enzyme (Figure 1).
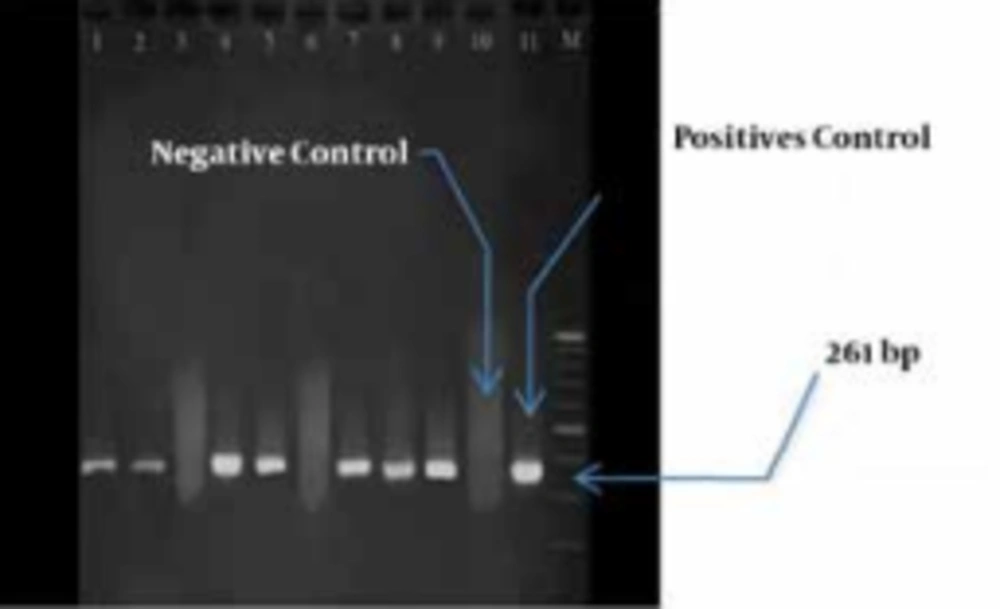
In the next step, PCR was conducted on nine isolates to evaluate the prevalence of IPM-1, SPM-1 and VIM-1 genes. It was found that seven isolates contained only VIM-1 gene, and the other genes were not detected. (Figure 2).
Among the isolates that contained VIM-1 gene, three samples were isolated from lesions, three from mid-stream urine and one from endotracheal tube (ETT). Totally, four isolates belonged to ICU. These seven isolates were resistant to all of the studied antibiotics, but they were sensitive to polymyxin B and colistin.
5. Discussion
Drug-resistant P. aeruginosa has caused a serious challenge to treat infections due to these bacteria. Different antibiotics including beta-lactamases, aminoglycosides and quinolones are applied to treat these infections (9). Antibiotic resistance pattern of the bacteria is changing quickly. However, studies show that the increasing tendency of resistance against antibiotics has some fluctuations especially in the case of imipenem (10). Since infections due to multidrug-resistant P. aeruginosa, with high membrane permeability, are stable against most of beta-lactamases, carbapenems are often used to treat them (11). However, in recent decades, there have been a lot of warnings about the increase in p.aeruginosa resistance against antimicrobial agents. These organisms have various mechanisms for self-protection against antibiotics. One of the most important mechanisms for resistance against imipenem is the production of metallo-beta-lactamase enzymes (12). Metallo-beta-lactamases of VIM, IMP and SPM families are quite prevalent in P. aeruginosa (13). Since various reports indicate the increased resistance of P.aeruginosa species to antibiotics (especially imipenem), proper use of these antibiotics and timely identification of species producing metallo-beta-lactamases should be considered. It causes successful treatment and prevents propagation of resistant genes which can be seriously harmful for societies (14). There are different methods to identify MBL-producing species. The present study used E-test tapes; these tapes contain EDTA which is a metallo-beta-lactam inhibitor (15). In the current study, seven out of nine isolates which were positive in E-test tape method (phenotypic method) ,contained VIM-1 gene and two isolates were negative in both phenotypic and genotypic methods (PCR), which indicated involvement of other factors such as lack of oprD (results in membrane permeability change), the presence of efflux pumps or chromosomal Ampc beta-lactamase. Two isolates which were positive in phenotypic method were reported negative in PCR, indicating that other genes were involved in this process. Among isolates containing VIM-1 gene, four species (57%) were isolated from patients hospitalized in ICU for approximately a long time and undergone antibiotic therapy, which indicates the presence and propagation of the mentioned gene under such circumstances.
In this investigation, IMP-1 and SMP-1 genes were not observed in any of the imipenem- resistant strains. In a study by Shahcheraghi et al. on 243 P.aeruginosa species isolated from Imam Khomeini Hospital in Tehran showed that 15 isolates contained VIM-1 gene. Also, Sepehri et al., reported that 44 out of 283 P. aeraginosa species isolated from lesions, contained VIM-1 gene (16). The results of the above researches are nearly consistent with those of the present study indicating that VIM-1 is the predominant metallo-beta-lactamase in Iran. Another research was conducted by Fazeli et al., in Isfahan and showed that 34 out of 111 (43%) of the isolates collected from Imam Musa Kazem Hospital carried VIM gene (17),and it is clear that the results of their research are not consistent with those of the current study. It can be said that the prevalence of metallo-beta-lactamase in the isolates collected from that hospital was more than expected. Since Imam Musa Kazem Hospital is a burn center, a large amount of imipenem is consumed there and resistance against imipenem has reached 94.9%. Therefore, it is not unexpected that genes driving drug resistance, especially metallo-beta-lactamase genes which are mostly located on dynamic elements (plasmid-transposon), are propagated more in this center.