1. Background
Patients colonized with methicillin-resistant Staphylococcus aureus (MRSA) are known to be at high risk of developing MRSA infections (1). Currently, MRSA infections are increasing in number and in the Japan Nosocomial Infection Surveillance report of 2011, the rate of MRSA in Staphylococcus aureus isolates was reported to be 54.6% (2).
In 2003, the increase in vancomycin minimum inhibitory concentration (MIC) became an important socio-economic issue as well as a threat to the health-care system (3). In 2006, the Clinical Laboratory Standard Institutes changed the breakpoint of the vancomycin MIC for MRSA (4). Currently, with the rise in the vancomycin MIC, the Infectious Diseases Society of America guideline recommends setting a higher vancomycin trough level at 15 - 20 µg/mL, but the data do not yet clearly demonstrate whether this higher trough level is associated with an improved prognosis (5). Recent report shows that higher vancomycin MIC levels are associated with a higher mortality rate in patients with MRSA bacteremia even with the use of anti-MRSA antibiotics (6). Higher-dose vancomycin regimens are also associated with a higher likelihood of vancomycin-related nephrotoxicity (7, 8).
In Japan, there is no data yet regarding MRSA colonization rates, nor an antibiogram of MRSA colonization. The antibiogram of MRSA, including MIC levels for MRSA colonization, is important to select appropriate empirical antibiotic therapy in critically ill patients. Our previous study showed that the MRSA colonization rate is 30% among patients in Japan at high risk for MRSA (9).
2. Objectives
The purpose of this study was to define the antibiogram for anti-MRSA antibiotics, including MIC levels, from MRSA colonized patients.
3. Patients and Methods
This study was approved by the Institutional Review Board of the St. Marianna university school of medicine. The study design, data collection, data analysis, and manuscript preparation were conducted without corporate support. The MIC was determined for MRSA isolates in high-risk patients between November 2009 and March 2011, seen in two tertiary care hospital emergency departments. Patients were screened for clinically significant risk factors for MRSA colonization as described in our previous study, and are summarized in Table 1 (9). Exclusion criteria included patients with the age of 18 years or less, or unwillingness to participate in this study.
| Seven Risk Factors of MRSA Colonization as Inclusion Criteria | Incidence, N (%) |
|---|---|
| 1) Past history of MRSA colonization | 13 (20.0) |
| 2) Hospitalization from a long-term care facility in the past 5 years | 22 (33.8) |
| 3) ≥ 30 days hospitalization in the past 6 months | 31 (47.7) |
| 4) End-stage kidney diseases on hemodialysis | 7 (10.8) |
| 5) Chronic skin diseases | 9 (13.8) |
| 6) Patients with malignant diseases with ≥ 1 factors among following 5 factors | 7 (10.8) |
| a) ≥ 5 hospitalizations in the past 1 year | 3 (4.6) |
| b) History of chemotherapy in the past 30 days | 4 (6.2) |
| c) History of surgical operation in the past 30 days | 0 (0) |
| d) History of antibiotics usage in the past 30 days | 4 (6.2) |
| e) Continuous Foley catheter placement | 1 (1.5) |
| 7) ≥ 2 factors among following 4 factors | 52 (80.0) |
| a) ≥ 75 years old | 44 (67.7) |
| b) History of hospitalization due to acute illness in the past 6 months | 48 (73.8) |
| c) History of following antibiotic usage (Quinolon Cepharosporin Carbapenem) in the past 6 months | 26 (40.0) |
| d) ≥10 days hospitalization in the past 3 months (6 months) | 49 (75.3) |
a Abbreviation: MRSA, methicillin-resistant Staphylococcus aureus.
Specimens for culture were obtained from patients’ nasal cavities within the first 48 hours of hospitalization. Sample collection was performed as previously reported (10). During the study period, resampling was not performed if they were previously determined to be MRSA-positive. However, resampling was obtained from patients who had not undergone sampling for more than one month and were confirmed MRSA-negative from any site of infection.
Clinical Laboratory Standard Institute guidelines (11) were used for detection of antibiotic resistance. The MIC of anti-MRSA drugs was evaluated by the E-test (SYSMEX bioMerieux Co. Ltd) and the micro-dilutional test using Microscan® (SIEMENS, CA, USA).
Arbekacin (Meiji Seika Pharma Co., Ltd) is one of the anti-MRSA medications available in Japan. The protocol for Arbekacin use is to check the MIC of the MRSA strain from the patient and then Cmax/MIC is set to achieve a goal of 8 - 10. When aiming to be effective against MRSA strains of MIC 2 µg/mL, Cmax is recommended to achieve a level of 15 - 20 mg/L (12).
The E-test was performed by evenly spreading the medium in three directions on a commercial agar plate. The medium was prepared to achieve the bacterial concentration (0.5 Macfarland) according to the turbidity standard technique. The broth microdilution method was performed by measuring the turbidity with Microscan. The microbiology laboratory initially performed bacterial isolation, and the isolates further tested with the E-test and broth microdilution methods. The E-test and broth microdilution tests were performed by people uninvolved with the study to minimize bias. The primary outcome was the value of the MIC of vancomycin and teicoplanin in isolated MRSA strains. A secondary outcome was the antibiogram of anti-MRSA antibiotics other than vancomycin and teicoplanin.
3.1. Statistical Analysis
StatFlex version 6.0 (StatFlex version 6.0, Artec Inc. Osaka, Japan) was used for statistical analysis. Vancomycin and teicoplanin MIC results using the E-test and broth micro-dilution method were compared using a t-test with parametric analysis.
4. Results
Clinical data for 276 patients were reviewed. Of these, 23.6% (65/276) had MRSA isolated from surveillance cultures. The clinical characteristics of these 65 patients are shown in Table 1.
When tested with the E-test, the average MRSA MIC values were 1.7 µg/mL and 2.1 µg/mL for vancomycin and teicoplanin, respectively. Using the E-test, the MIC50 and MIC90 of vancomycin for MRSA isolates were both 2 µg/mL, the MIC50 for teicoplanin for the MRSA isolates was 2 µg/mL, and the MIC90 was 3 µg/mL. When evaluated with the broth microdilution method, the average MRSA MIC was 1.2 µg/mL for vancomycin and 2.1 µg/mL for teicoplanin.
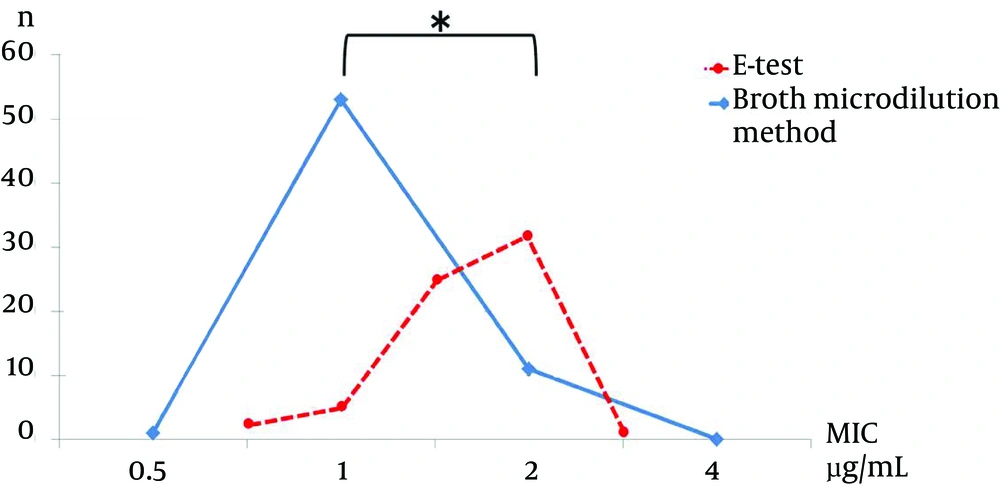
The MRSA MIC results for vancomycin and teicoplanin were as follows. For vancomycin, the MIC using the E-test showed that 3.1% (2/65) of strains had a MIC of 0.75 µg/mL, 7.7% (5/65) had a MIC of 1 µg/mL, 38.5% (25/65) had a MIC of 1.5 µg/mL, 49.2% (32/65) had a MIC of 2 µg/mL, and 1.5% (1/65) had a MIC of 3 µg/mL. The vancomycin MIC result using the broth microdilution method showed that 1.5% (1/65) had a MIC of 0.5µg/mL, 81.5% had a MIC of 1 µg/mL, and 16.9% (11/65) had a MIC of 2 µg/mL (Figure 1).
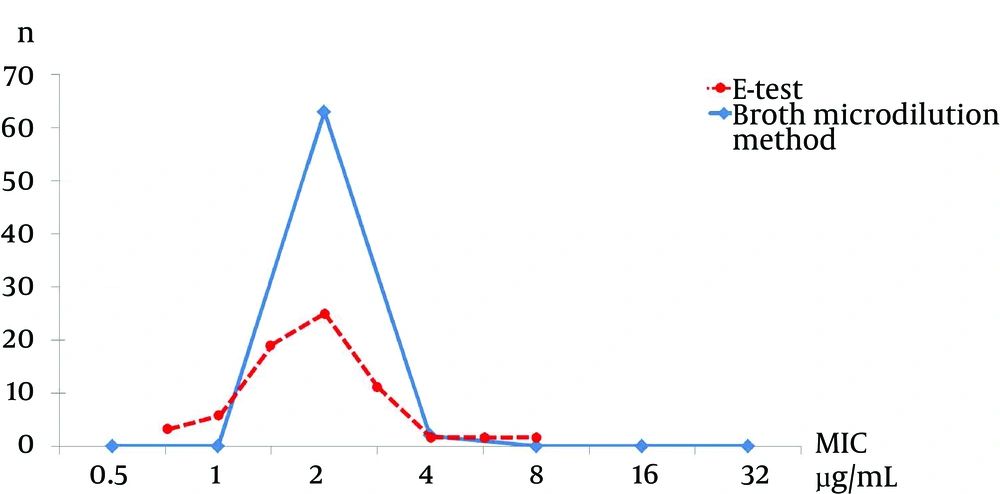
The MIC for teicoplanin using the E-test showed that 4.6% (3/65) of strains had a MIC of 0.75 µg/mL, 9.2% (6/65) had a MIC of 1 µg/mL, 27.7% (18/65) had a MIC of 1.5 µg/mL, 36.9% (24/65) had a MIC of 2 µg/mL, 16.9% (11/65) had a MIC of 3 µg/mL, and 4.5% (3/65) had a MIC ≥ 4 µg/mL. The teicoplanin MIC using the broth microdilution method showed that 96.9% (63/65) had a MIC below 2 µg/mL and 3.1% (2/65) had a MIC of 4 µg/mL (Figure 2).
When we compared the MIC results from the E-test and broth microdilution methods, we found statistically a significant difference for vancomycin (P < 0.001), but not for teicoplanin (P = 0.81).
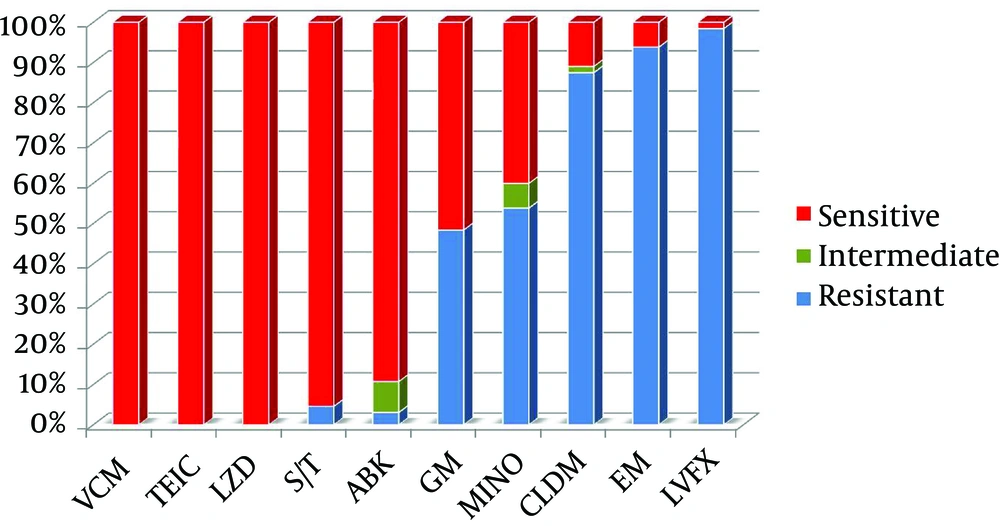
All MRSA strains isolated in this study were sensitive to linezolid. The linezolid MIC using the broth microdilution method showed that 87.7% (57/65) had a MIC ≤ 2 µg/mL, and 12.3% (8/65) had a MIC 4 µg/mL. The sensitivity of other anti-MRSA antibiotics is shown in Figure 3. A total of 95.4% (62/65) of the MRSA isolates were sensitive to trimethoprim/sulfamethoxazole. Of the MRSA isolates, 47.7% (31/65) were gentamicin sensitive, 40% (26/65) were minocycline sensitive, 13.8% (9/65) were clindamycin sensitive, 6.2% (4/65) were erythromycin sensitive, and 1.5% (1/65) were levofloxacin sensitive.
5. Discussion
According to previous reports, the MRSA colonization rates for ER and ICU patients in Japan were 2.9% and 11.2%, respectively (13, 14). Reports regarding the MIC for MRSA isolates in MRSA colonized patients are lacking. The results of the present study show that the MIC level for vancomycin is higher than 1.5 µg/mL in MRSA colonized patients. This result is potentially of great significance in the situation when an MRSA colonized patient, known to be at high risk for an MRSA infection, shows signs and symptoms of infection and needs empirical anti-MRSA treatment. In these patient populations, it is anticipated that treatment with vancomycin may not be sufficient to adequately treat MRSA infection, and other anti-MRSA antibiotics may need to be considered.
There have been several reports comparing the efficacy of vancomycin with linezolid in treating nosocomial pneumonia and MRSA pneumonia (15, 16). A double blinded randomized controlled trial by Wunderink and colleagues showed that there was no statistical difference in 60-day mortality between the vancomycin and the linezolid groups, but showed favorable clinical and microbiologic responses in the linezolid group (16). This report, however, has some limitations. In particular, the group treated with vancomycin included more severely ill patients, and the difference in clinical outcomes was not statistically evident in intention-to-treat analysis, but only in per protocol analysis. There has also been a report of an outbreak of linezolid resistant MRSA strains (17). A report in 2012 comparing daptomycin and vancomycin in treating blood stream infection with MRSA and a vancomycin MIC > 1 µg/mL, showed that daptomycin has greater treatment efficacy and fewer side effects (18). This suggests that there will be ongoing debate regarding the use of anti-MRSA antibiotics other than vancomycin when treating infections caused by MRSA strains with vancomycin MIC ≥ 2 µg/mL.
The mortality rate associated with MRSA bacteremia was significantly higher when the empirical antibiotic was inappropriate and when vancomycin was empirically used for treatment of infections with MRSA strains having a high vancomycin MIC (> 1 µg/mL) (6). A vancomycin MIC ≥ 2 µg/mL for MRSA infections has a high risk of treatment failure for MRSA infections (6).
We did not find a significant difference in teicoplanin MIC results when comparing the two methods used, the E-test and broth microdilution methods, but there was a statistically higher result for the vancomycin MIC result when determined with the E-test compared to the broth microdilution method. This difference may be explained by the fact that, in general, the E-test is reported to have a higher MIC result compared to the broth microdilution method when testing other antibiotics (19). In addition, most teicoplanin MIC results show an MIC ≤ 2 µg/mL, which suggests that the actual MIC result distributes in the lower ranges for MIC levels. As a result, the vancomycin MIC using the E-test seemed to correlate with the vancomycin MIC using the broth microdilution test; however, teicoplanin MIC levels measured using the broth microdilution method distribute to a lower range of teicoplanin MIC levels than the teicoplanin MIC level measured by the E-test. Not only in the present study, but also in other reports (19), it has been suggested to be problematic since there is a difference in MIC levels measured by the E-test and the broth microdilution test.
The broth microdilution method using Microscan®, used widely in Japan, has two methods for determination, including the the turbidity standard technique and the prompt system. The prompt system has a tendency to have higher bacterial content in the sample and is generally less reliable. Therefore, the turbidity standard technique is considered to be the gold standard for measuring the MIC for MRSA; however, MIC measurements using the turbidity standard technique may yield lower results than MIC measurements using the prompt system. As previously described (6), a higher MIC level is associated with a higher mortality rate, suggesting that the MIC should be measured using the E-test and the higher MIC level should be defined as an MIC ≥ 2 µg/mL. In a metanalysis (20), infections with MRSA strains with an MIC ≥ 2 µg/mL are associated with a significantly higher mortality rate. These results should be studied to evaluate the accuracy of MRSA MIC measurement using the E-test and the broth microdilution method as the broth microdilution method can underestimate the MRSA MIC of vancomycin and/or teicoplanin.
This study has several acknowledged limitations. Since the data was collected from two institutions, the results may not be generalizable to other sites. In addition, follow-up of MRSA colonized patients was not performed; thus, the long-term clinical implications for patients colonized with higher vancomycin MIC strains of MRSA should be studied further. Patients in this study are at high risk and may be different from typical patients colonized with MRSA. Therefore, the MIC could be higher compared to the MIC level of typical patients colonized with MRSA.
In two metropolitan tertiary hospital emergency departments, trimethoprim /sulfamethoxazole was the second most effective anti-MRSA oral antibiotic in vitro. This is the first study to show that MIC50-MIC90 of vancomycin for MRSA isolates from MRSA colonized patients was 2.0 µg/mL. These results may help to select appropriate empiric MRSA antibiotics for patients anticipated to be at high risk for treatment failure and nephrotoxicity if glycopeptides were used.