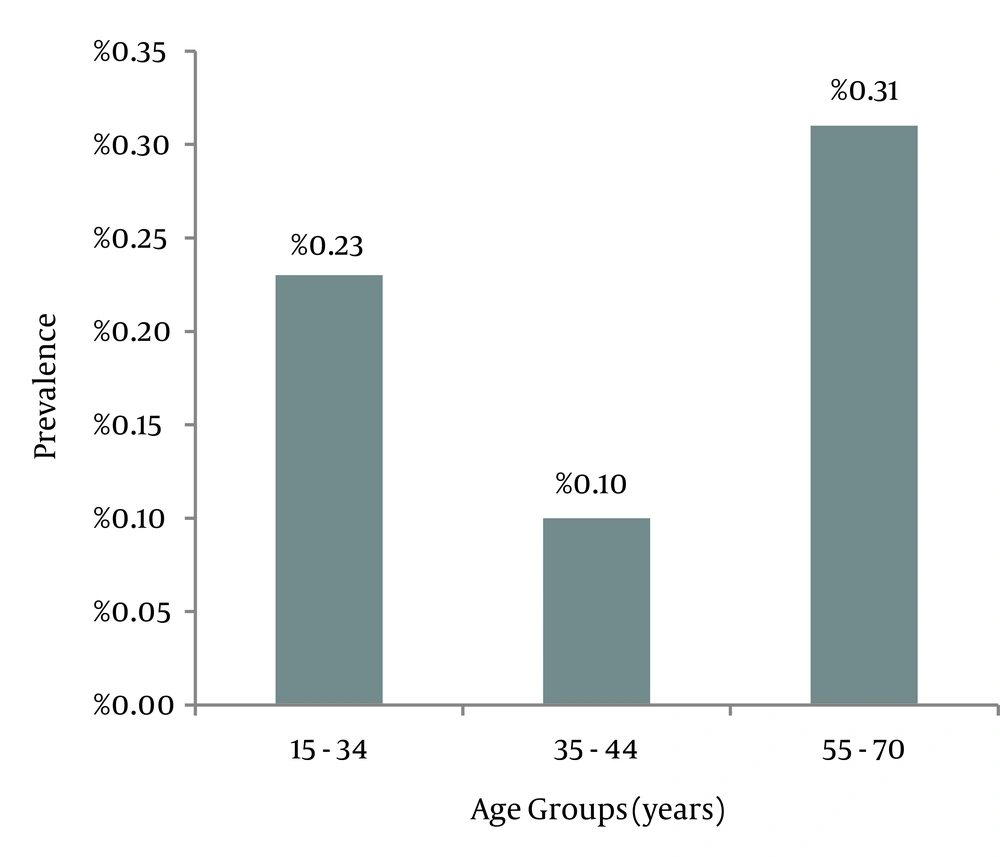1. Background
Hepatitis C virus (HCV) is an enveloped, single-stranded RNA virus that represents a major health problem in the world and is prevalent among chronic liver diseases. It has been estimated that HCV accounts for 27% of cirrhosis and 25% of hepatocellular carcinoma (HCC) worldwide (1).
The estimated prevalence of HCV infection is 2.2%, or about 170 million positive persons around the world. One study showed that prevalence ranges from a low of < 1% in Scandinavia in northern Europe (0.01 – 0.1) to a high of > 3% in Egypt in northern Africa (15 – 20%) (2). Risk factors associated with HCV are the transfusion of blood and its products, the use of injected street drugs or IVDU, accidental injections, birth to an infected mother, sexual contact, and illicit sexual activities (2). Among that population, 48.4% were IV-drug users. In a 2002 survey, the prevalence of HCV-Ab was 6.3% among people with tattoos.
In China, the prevalence rate ranged from 1% in the region of lowest endemicity to 31.86% (3) in the region of highest endemicity. The prevalence in Iran’s neighboring countries is 1.1% in Yemen (4), 1.8% Saudi Arabia (5), and 4% in Pakistan (6). In Iran, in the general population, the prevalence of HCV is 1% (7) and lower than upper countries. The prevalence of HCV infection in Iranian hemophilic patients was 15% in Fars, 44.3% in Kerman, 29.6% in Zahedan, 59.1% in Hamadan, 10 and 71.3% in Guilan. In another study, the prevalence of HCV in thalassemia patients was 19.3% (8).
A study conducted on hemodialysis patients showed a decreased prevalence of 4.5% for HCV-Ab in 2006 (9) compared to 14.4% in 1999 (9). Anti-HCV-Ab in Tehran dialysis patients was 19.5% (10, 11). A study conducted in 2013 on the general population of Mashhad (3870) showed Anti-HCV+ prevalence was 0.2% with ELISA and 0.3% with the PCR method (12).
2. Objectives
The aim of the study is to determine the prevalence and risk factors of HCV hepatitis among the general Birjand population.
3. Patients and Methods
This cross-sectional study was done to determine the prevalence of HCV in Birjand city. To determine the prevalence of HCV, 5,235 people were chosen, all older than 15 years, based on the cluster randomized method. The sampling method was a multistage kind, where 250 cluster heads were randomly chosen from the Birjand city post office, and from each head, 20 samples were chosen. From each head, 20 people in 5 age groups, from 15 to 69 years, were selected so that each age group included 2 women and 2 men. A checklist was designed according to the study variables and patients’ blood sample results. After determining the population, the authors visited the patients’ houses; after introduction of the study, permission and testimonials were obtained. The patients were invited to come for diagnostic HCV virus serology, and the data of blood sampling were given to them over phone. They were asked to come to the study center building on the day of the test. After the questionnaires were completed, risk factors for HCV were identified, including blood transfusion, familial history of HCV infection, or special encountering. Fasting blood samples of 5 cc were collected from each person and sent to a lab for determining the serology, and no anticoagulation substance was added. Sampling was done by skilled technicians at the site. The samples were transported to the lab immediately, and serums were stored in a freezer at –10°C until testing. After blood sampling, the titer and level of anti-HCV Ig was determined in each sample using HCV-Ab (DIA pro) ver. 4 enzyme immunoassay with a specificity of 99.5% and sensitivity of 100%. The test results and medical recommendations were given to the patients over the next 7 days. After specification of the test results, the data collection was done, and then data were entered into SPSS software by using statistical tests (percent, prevalence, and mean and standard deviation). To analyze the data, a chi-square test and Fisher’s test in an alpha level of 0.05 were used. Odd ratio (OR) also was estimated.
4. Results
This study was performed on 5,232 people in Birjand city; 47.8% of them were male and 52.2% were female. The mean age was 39.7 ± 14.4 (ranging from 15 to 70) (Figure 1); 81.1% of patients were married and 16.7% were single; others were widowed or divorced (2.2 %).
Among those with risk factors, 7% were smokers, 2.7% were non- IV-drug abuser addicts, 0.2% were IV-drug abusers, and 0.7% were addicted to alcohol. Familial history of HCV was seen in 0.4% of cases.
General prevalence of HCV (HCV-Ab) was 0.2% (10 cases). Among these, 6 patients (0.2%) were males and 4 were females (0.1%). Six patients (0.6%) had a primary school degree, and 4 were about 25 to 34 years old, but there was no significant relationship between prevalence of HCV-Ab and age, sex, and education.
Four cases each had a history of going to a dentist or having major surgery, and 7 cases had a history of hospitalization for more than 10 days. However, no significant relationship was found between the prevalence of HCV-Ab and histories of blood transfusions, going to a dentist, having surgery or an endoscopy, alcohol drinking, Therapeutic bloodletting, or smoking (P > 0.05).
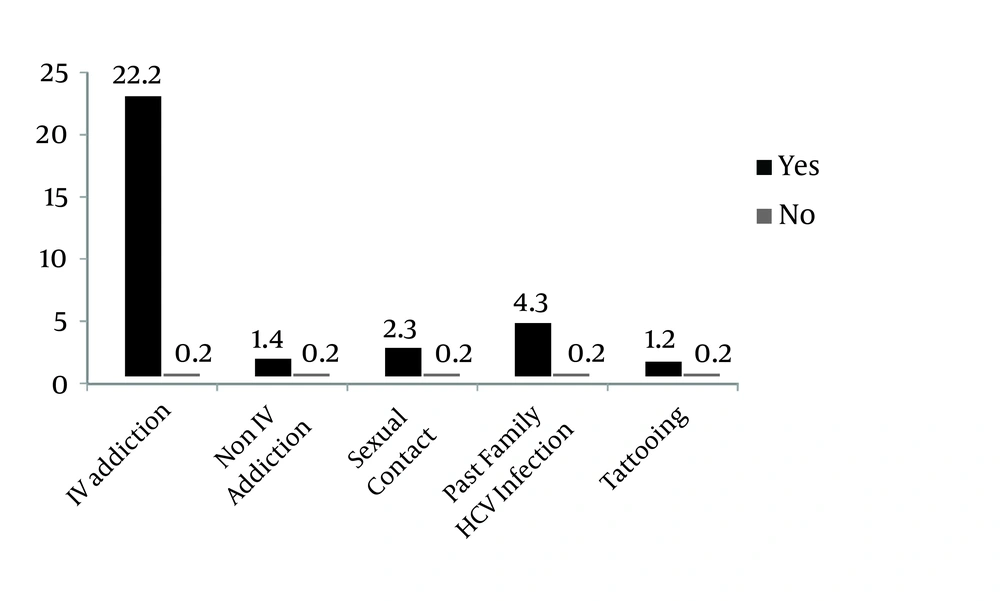
Seven cases were confirmed by PCR (5 cases were determined to be 3A, and 2 cases were 3B), so the prevalence of HCV (after PCR) was 0.14%, and the prevalence of HCV-Ab in non-IV-drug abuse addicts was 9.3 times more than in non-addict patients. In IV-drug abusers prevalence was 200 times higher. The prevalence of HCV-Ab patients with illicit sexual activities was 13.3 times more than in other patients without this risk factors , and prevalence among patients with a familial history of HCV infection was 26.3 times more than in other patients, which was significant (P < 0.001) (Figure 2).
5. Discussion
This study was conducted among 5,235 people to determine the prevalence and risk factors of HCV infection in Birjand city, the capital of South Khorasan.
HCV-Ab was detected by ELISA tests, which had positive rate of 0.2%, and the same was confirmed by PCR. Out of 10 patients (6 men and 4 women) who were HCV-Ab+ (0.2%), 7 patients (4 men and 3 women) were determined to be HCV-Ab+ with PCR (0.14%).The results showed that studies on the prevalence of HCV infection in Iran have mostly focused on high-risk people; for example, the study conducted by Alavian (2003) in the Iran Hemophilia Institute showed the prevalence of HCV-Ab was 60.2% (9).
There was no significant relationship found between HCV-Ab+ and sex, age, marriage, or kind and severity of bleeding disorder. The kind and numbers were determined by coagulative factors and familial history of hepatitis. Also, there was no relationship found between HCV-Ab+ and Hepatitits B Antigen positivity (HBS-Ag+), but the prevalence of HCV-Ab was significantly higher among HBS-Ag+ than HBS-Ag negative patients. In a study conducted in Tehran on 202 IV-drug abusers in 2006, the prevalence of HCV-Ab was 52%. During the nine years (1994 – 2008) of the study in Iran, 19.3% of the patients with thalassemia were HCV-Ab+. Also, 3.1% of immigrants were HCV-Ab+ (13). In a study conducted on IV-drug abusers in Hamedan, the prevalence of HCV-Ab was 31 – 47%, and was 31.5% among prisoner IV-drug abusers. Also, 45.4% of 460 prisoners were HCV-Ab+ in Gilan (7).
In a study conducted among 15,252 blood donors in 2003 in Kerman, 0.39% of them had a definite positive result for HCV, and there was no significant relationship found between HCV infection and age or marriage (14).
A study was performed by Alavian and Fallahian in 200910 to determine the prevalence of HCV infection in Iran; it showed that the prevalence of HCV-Ab was about 15.6% in Iranian hemophils in Fars, 44.3% in Kerman, 29.6% in Zahedan, 59.1% in Hamedan, 71.3% in Guilan, and 50% overall in Iran (7).
Among dialysis patients in Tehran, the prevalence was 19.6%, and the prevalence of the disease become noticeably lower (from 14.5% in 1999 to 4.5% in 2006) in Iran (9). In other countries, the prevalence of the disease in special groups is higher. For example, in Vietnam, 29% of hemophilia patients were HCV-Ab+ in 2010 (15).
In a study conducted in Egypt in 2008, it was reported that the prevalence of HCV-Ab in blood donors was 26% (16), and a study done in Saudi Arabia on 1,214 patients suffering from viral hepatitis during 2005 showed that HBV infection is the most common type of viral hepatitis, and HCV, at 60.7%, is the second most common type (5).
A study in England showed that hemophilia patients with HCV-Ab were 83% in 1997, and were 84.28% in 1998. This number was 40% in the United States in 1999 (17).
A study conducted on 12,167 blood donors in Thailand in 2004 showed a HCV-Ab+ rate of 29% among them; in England 7.8% of the donors were positive. Some studies have been conducted recently on the worldwide population.(18, 19) For example, a study to determine the epidemiology of HCV in 2014 showed that the prevalence of HCV-Ab is 2% for adults and 1.6% for all ages all over the world. The prevalence of viremia is 1.4% in adults and 1.1% in all ages.
China with 1.3%, Pakistan with 6.7%, Nigeria with 8.4%, Egypt with 14.7%, and Russia with 0.25%; and in total 25%, it has over half of the HCV-infected cases (20).
A study conducted on 3,870 people in Mashhad during 2012 that showed a prevalence of 0.2% for HCV-Ab, and that 0.13% of those were confirmed by PCR (12).The prevalence of HCV showed in this study is in accordance with the Mashhad and Iran studies.
In the authors’ study, 52.2% of the population were female, 29.9% had a university degree, and there was no significant relationship found between HCV-Ab and age, sex, or education level (P > 0.05). Also, a significant relationship was found between HCV-Ab and risk factors such as blood transfusion, alcohol drinking, smoking, endoscopy, or surgery (P = 0.2).
Compared to non-addict patients, HCV- Ab was 200 times more prevalent in IV-drug abusers and 9.3 times more prevalent in non-IV-drug abusers (P < 0.001); also, prevalence was 13.3 times higher in patients with illicit sexual activities compared to patients with no such history (P = 0.001). The prevalence in patients with familial history of the disease was 26.3 times higher than in patients with no familial history (P ≤ 0.04), and hence the relationship was significant.
HCV is among diseases that can be transferred by blood, IV-drug abusing, illicit sexual activities, and medical processes such as endoscopy, colonoscopy, or hemodialysis. Data gathered by checking and screening blood donors for HCV shows that IV-drug abusing is the most common way of transferring HCV infection, accounting for 60% of all chronic and new infections (1).
A study was conducted on 1,997 volunteers from June 2005 to November 2007 in Kualalampur to determine the prevalence of HBV and HCV infection, which showed that 0.2% were HCV-Ab+ and 0.3% were both HCV-Ab+ and HBC-Ab+. Among those, 24.8% were IV-drug abusers, and 13% of the patients had a history of hepatitis (21).
Another study conducted on 884 volunteers in Bangladesh showed a prevalence of 22% for HCV-Ab, and IV-drug abuse was a risk factor in 60% (22). Further study among 6,917 patients in 2010 in Spain determined that of 195 patients with HCV-Ab+, 75.9% were positive based on PCR, and 75% were viremic (23). A similar study in 1995 involving 2,935 people in Australia showed that 86.4% of patients with HCV infection were IV-drug abusers (24).
In another study conducted in Bolivia in 2005, IV-drug abuse was an important risk factor for HCV, and 90% of patients with HCV had one of the following risk factors: (25) IV-drug abuse, sexual intercourse with infected people, multiple partners, family history, Sexual transmitted disease (FSTD), and blood transfusion. Hence, IV-drug abuse is the most important risk factor, and other risk factors, such as medical procedures, are less frequent due to measures taken to prevent contamination. After IV-drug abuse, notable risk factors are illicit sexual activities and multiple sexual partners. A separate small study involved genotyping, but due to the small size of the sample, a relationship could not be determined between genotype and the risk factors and demographic specifications. Ten patients with HCV-Ab+ in this study included 5 patients between 25 and 34 years old. Patients with both HCV-Ab+ and PCR+ underwent genotyping; 4 patients out of 7 were 3a, and the other 3 patients were 3b.
Also, the patients had IV-drug abuse risk for HCV infection, which accompanies with genotype 3a in most of the studies (26). For example, a study conducted in 2007 in France showed that genotype 1a accompanies with transfusion risk, and genotype 3a accompanies with IV-drug abuse risk (27). In another study, most of the HCV infected patients younger than 45 years had genotype 3a, and other genotypes were seen in those older than 45 years. The increased prevalence of 3a patients in this study is in accordance with the above-mentioned studies (28).
The present of prevalence of HCV in Birjand city is lower than Iran’s average, and prevalence of HCV infection is directly connected to IV-drug abuse.