1. Background
Urinary tract infections (UTIs) remain the most common bacterial infection among human. They are also one of the most frequently occurring nosocomial infections. They represent about 40% of all nosocomial bacterial infections worldwide (1). They are one of the leading causes of morbidity and mortality in the first 2 years of human life, in women and mostly in elderly with huge financial implications associated (2, 3). UTIs are acquired by various mechanisms. However, 70 to 80% of these infections are attributable to the use of an indwelling urethral catheter (4). Moreover, catheter-acquired urinary infection is the source of about 20% of episodes of health-care acquired bacteremia in acute care facilities. It is over 50% in long-term care facilities (4). Additionally, the use of indwelling urethral catheters increases the risk of UTI occurrence in health-care settings 14-fold (5). Recent prevalence studies around the world have reported that a urinary catheter is the most common indwelling device, with 17.5% of patients in 66 European hospitals having a catheter and 23.6% in 183 US hospitals (6, 7). These figures show that urethral catheters are commonly used in healthcare facilities and therefore the control of infections attributable to these devices should be an important goal of healthcare infection prevention programs. Furthermore, a huge variety of microorganisms are involved in UTIs (8).
However, the high prevalence of multidrug resistant bacteria worldwide could complicate the therapeutic process against these infections. Therefore, a periodical surveillance study is needed at national and regional levels to address the effectiveness of antibiotics being used and update the antibiogram of the associated microorganisms.
In Benin, limited statistics are available on catheter-associated UTIs. This paradox is surprising, as these are real phenomena affecting patients admitted at the hospital or taken care of at home. The aim of the present study is to provide scientific information about this important health challenge and to improve the care of catheterized patients at a hospital in Zinvie.
2. Objectives
This study aimed to: 1) Determine the prevalence of UTIs among catheterized patients, 2) Assess the sensitivity of isolated bacteria to the antibiotics, 3) Investigate the hygiene practices of the staff toward the risks of the occurrence of UTIs in the catheterized patients at the hospital.
3. Patients and Methods
3.1. Study Area and Design
The study was designed as a descriptive prospective cross-sectional study conducted within a hospital in Zinvie on catheterized patients. The services of the Emergencies, Medicine, and Surgery Departments of the hospital served as sampling areas. The bacteriological analyses were carried out at in the Bacteriology Laboratory of the hospital.
3.2. Material
Apart from the checklist used in direct observation to investigate the hygiene practices during catheterization, various other materials were used. Sterile syringes were used to collect urine samples, and conventional bacteriological culture media were used for the identification of bacteria according to Meite et al. (9).
3.3. Reagents
Many reagents were used: latex agglutination tests for the identification of S. aureus, oxidase, Gram-staining reagents (crystal violet, Gram’s iodine, alcohol and the fuchsine), oxygenated water for the catalase test, the Api 20E gallery for the identification of enteric bacteria, and antibiotic disks (Table 1) for the sensitivity test.
| Bacteria/Antibiotics Used | Symbols | Content, µg |
|---|---|---|
| Bacilli | ||
| Ampicillin | AMP | 30 |
| Amoxicillin | AMX | 30 |
| Cefotaxime | CTX | 30 |
| Ceftriaxone | CRO | 30 |
| Ciprofloxacin | CIP | 05 |
| Gentamicin | CN | 30 |
| Cocci | ||
| Cefoxitin | FOX | 30 |
| Erythromycin | E | 30 |
| Pristinamycin | PT | 30 |
| Tetracycline | TE | 30 |
| Vancomycin | VA | 30 |
List of Antibiotics Used
3.4. Methods
Based on the preliminary study by Konan (1995), which focused on 30 patients surveyed, the theoretical sample size was increased to 60. Thus, the examination of urine and susceptibility testing were carried out on 60 urine samples. These samples were collected from patients with urinary catheters. They were hospitalized in the department of emergency, medicine and surgery.
3.5. Inclusion Criteria
Cytobacteriological analyses of urine were carried out in patients with the following criteria:
- Be a hospitalized patient at the Hospital during the study period and scheduled for a catheterization
- Give clear oral consent or obtain consent from of the patients’ relatives in cases of comatose patients.
3.6. Culture Media Preparation
Culture media were prepared according to the manufacturer’s instructions.
3.7. Samples Collection
Urine samples were taken from catheterized patients as follows:
The catheter was clamped for 10 minutes to let urine accumulate upstream. Using alcohol, the clamp was disinfected to collect the intra-luminal urine of the catheter with a sterile syringe of 10 cc. Urine samples were aseptically collected 10 minutes following catheterization on day D1 and 48 hours later on day D3. Each sample was followed by the hygiene observation checklist and patients’ information, including names, sex, and age.
3.8. Laboratory Manipulations
The isolation of bacteria was performed on common bacteriological culture media, such as EMB, MacConkey, Chapman, Mueller Hinton, and CLED agars. Bacteria were identified according to conventional biochemical techniques (9). The gallery Api 20E was used to identify Gram-negative bacilli, while catalase, oxidase, and latex tests were used for the identification of the Gram-positive cocci. The resistance profile of the bacteria was established through the realization of their antibiogram. The disk diffusion method was used. The interpretation of inhibition zone diameters was made according to the recommendations of the antibiogram committee of French microbiology society (10).
3.9. Statistical Analyses
Data were stored in Microsoft Excel 2013, whereby various proportions were calculated. Using the Chi2 or Fisher’s test (depending on the sample size) in EPI-INFO 7, proportions of UTI-positive patients were compared based on sex, reasons for hospitalization and hospital section. Different species of bacteria isolated during the study period were sorted out and proportions of isolation of each bacterium were compared to assess the most predominant species involved in UTIs.
The results were considered statistically significant at 95% and presented as tables and graphs.
4. Results
4.1. Characteristics of the Studied Population
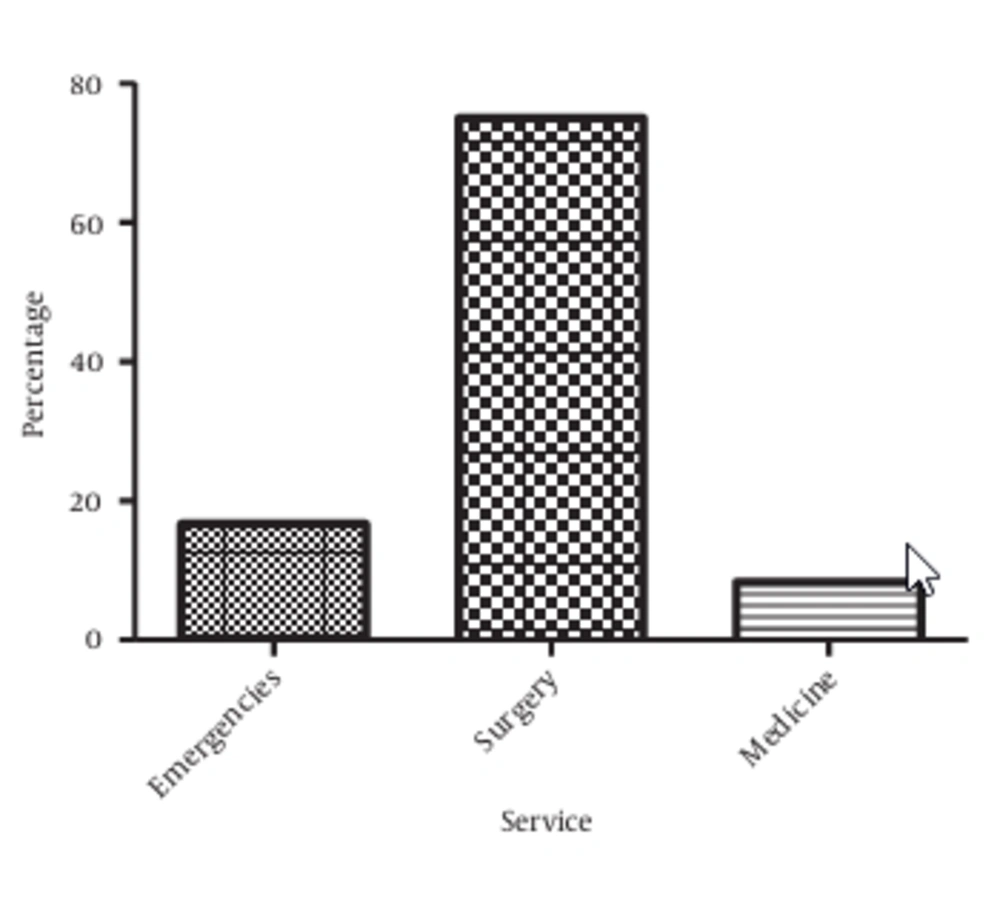
The patients were hospitalized in three different services. Surgery provided the highest proportion of catheterized patients (75%), followed by Emergencies (16.67%) and Medicine (8.33%) (P < 0.05, Figure 1).
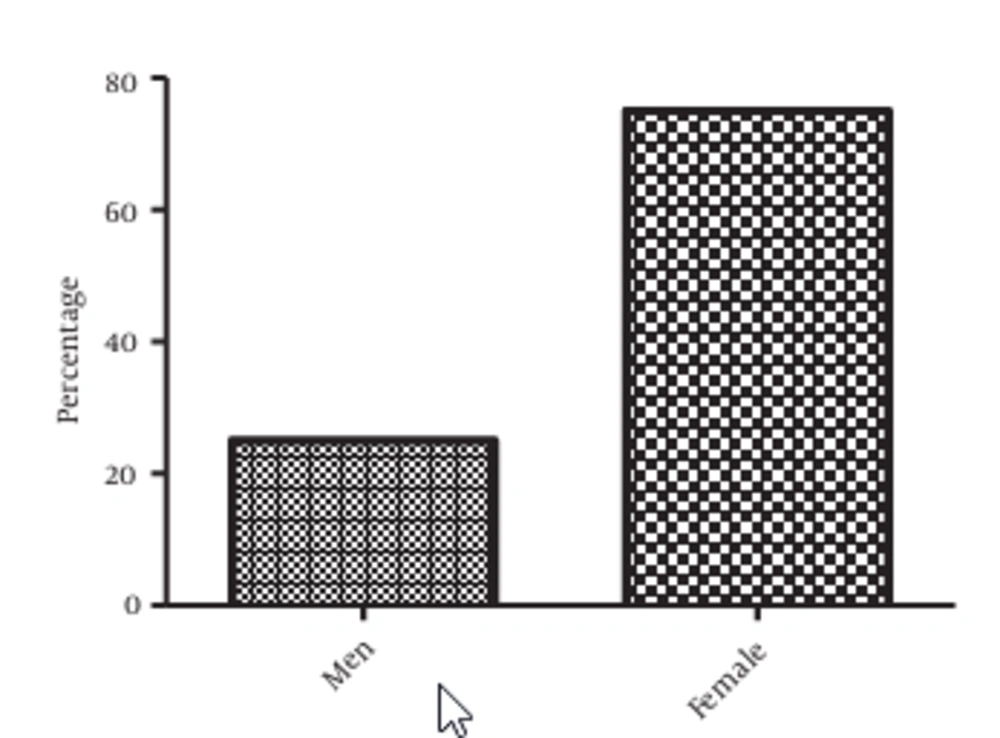
Regarding sex, 75% of the studied population are women and 25% are men (Figure 2). A significant difference was observed between these proportions (P = 0.000001), meaning that there are more catheterized women than men in the studied population.
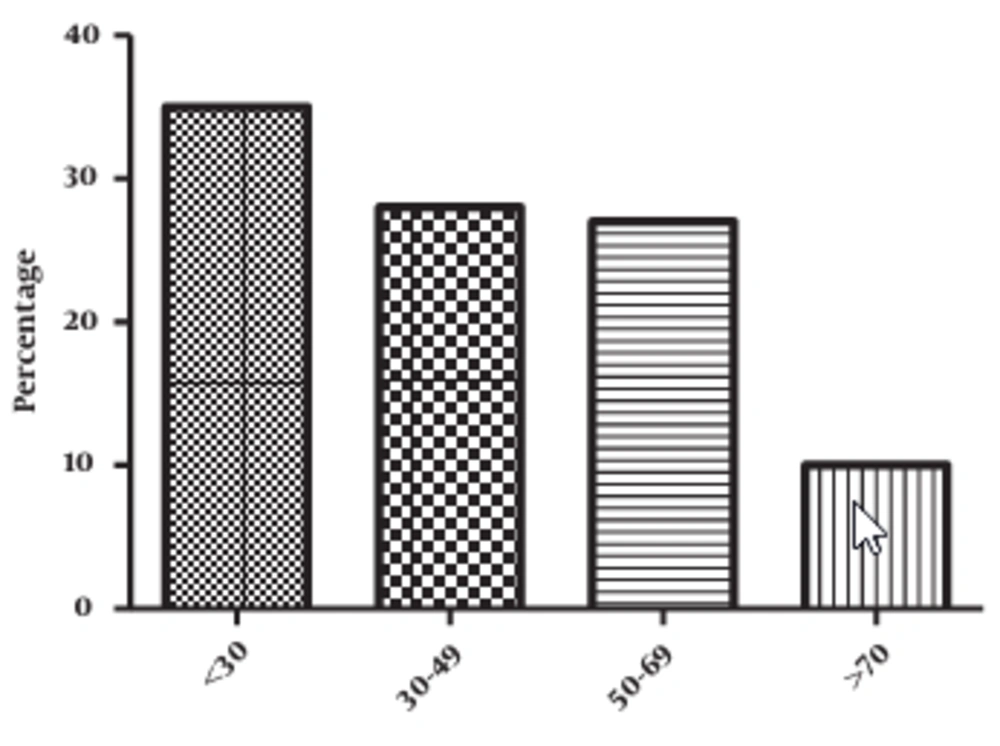
The mean age of the studied population was 56 ± 2 years with extremes going from 14 to 90 years. Patients less than 30 years of age represent 35% of the studied population (Figure 3).
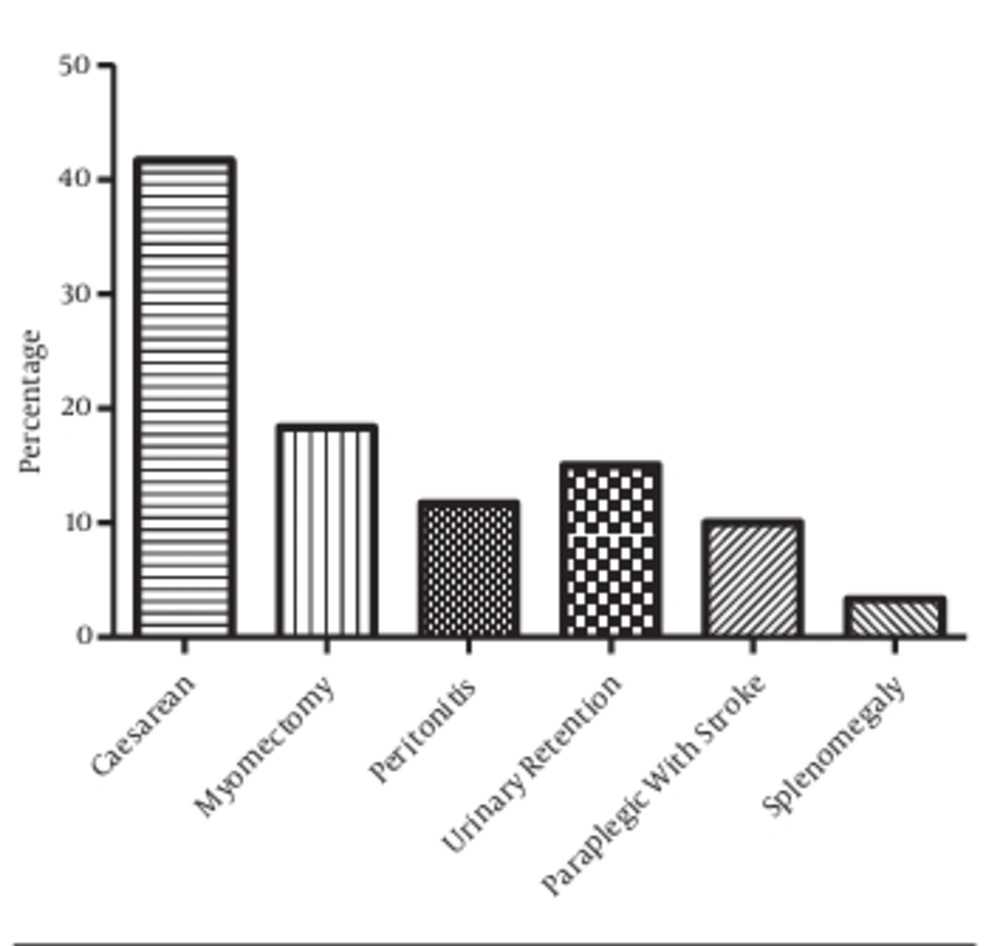
Six pathologies were the causes for the catheterization of patients in the study population. These include cesarean delivery (C-sections) (41.67%), myomectomy (18.34%), peritonitis (11.67%), urinary retention (15%), paraplegia (10%), and splenomegaly (3.33%). However, caesarean delivery represent the first pathology leading to catheterization in the studied population (P < 0.05), followed by myomectomy and urinary retention. The proportion of catheterized patients due to splenomegaly is significantly lower than those of caesarean delivery and myomectomy (P < 0.05). Furthermore, caesarean delivery, myomectomy, and urinary retention represent 75% of all pathologies that required catheterization in the study population (Figure 4).
The most frequent pathologies leading to catheterization in women were caesarean delivery and myomectomy while urinary retention was the leading pathology in men (Table 2).
| Pathology Leading to Catheterization/Gender | Number | Proportions, % |
|---|---|---|
| Caesarean delivery | ||
| M | 0 | 0 |
| F | 25 | 41.66 |
| Myomectomy | ||
| M | 0 | 0 |
| F | 11 | 18.34 |
| Peritonitis | ||
| M | 4 | 6.66 |
| F | 3 | 5.00 |
| Urinary retention | ||
| M | 7 | 1.66 |
| F | 2 | 3.34 |
| Paraplegia with AVC | ||
| M | 4 | 6.66 |
| F | 2 | 3.34 |
| Splenomegaly | ||
| M | 0 | 0.00 |
| F | 2 | 3.34 |
| Total | ||
| M | 15 | 25.00 |
| F | 45 | 75.00 |
Distribution of Catheterized Patients According to Sex and Pathologies
4.2. Hygiene Practices Among Health Personnel
Overall, there is a uniformity in the catheterization technique used in the three departments. The same hygiene practices were observed in all these services, apart from surgery, in which patients washing is practiced before the insertion of the catheter. The sole common hygiene measure of the three services is wearing gloves before the operation (Table 3).
| Departments | Observed Parameters | ||||
|---|---|---|---|---|---|
| Hands Washed Before Catheterization | Wearing Gloves Before Catheterization, % | Patient Washing Before Operation, % | Disinfection of the Operation Surface, % | Asepsis of the Catheter, % | |
| Emergencies | 0 | 100 | 0 | 0 | 0 |
| Medicine | 0 | 100 | 0 | 0 | 0 |
| Surgery | 0 | 100 | 100 | 0 | 0 |
Distribution of the Services According to Hygiene Practices
4.3. Bacteriological Risks
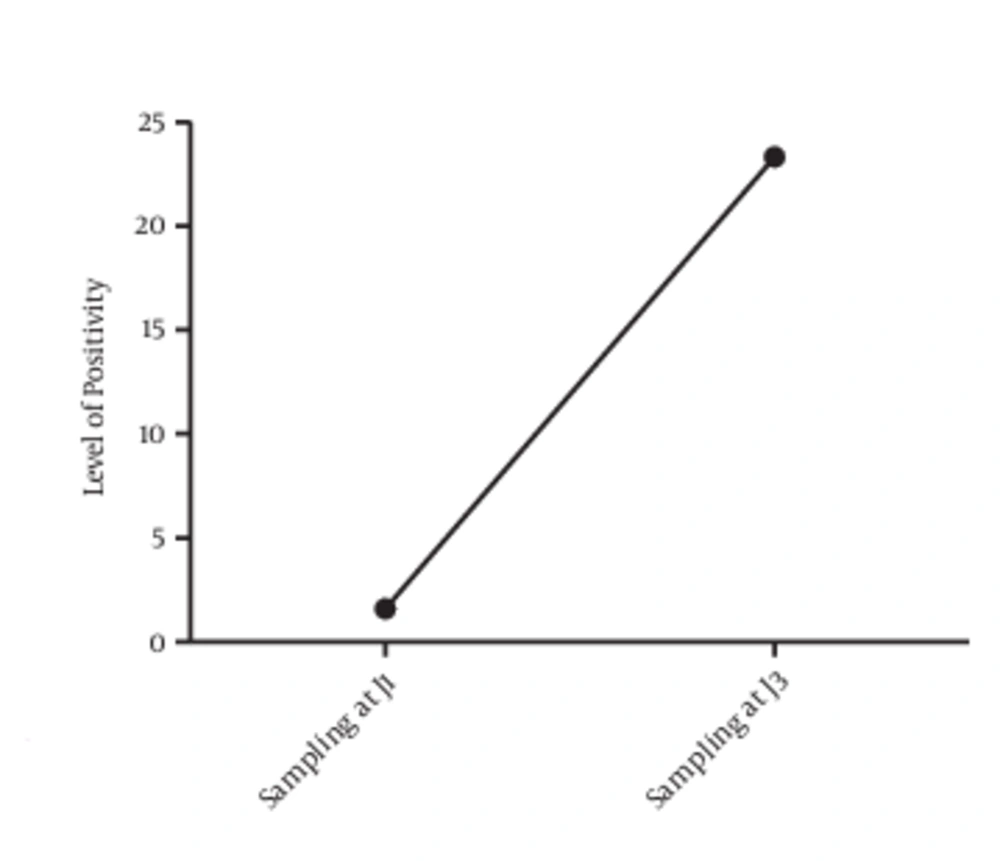
Out of the 60 patients sampled the first day, only one presented with bacteriuria (1.6%). Two days after catheterization, 13 non-affected patients on the first day presented with an infection in addition to the first initially infected patient. The prevalence of catheter-associated UTIs is 1.6% on the first day and 23.33% on the third day, with 14 positive cases (Figure 5).
Among the infected patients two days after the operation, there were six men and eight women (Table 4).
| Culture | Sex | Total | |
|---|---|---|---|
| Men | Female | ||
| Positive | 6 | 8 | 14 |
| Negative | 9 | 36 | 45 |
| Total | 15 | 44 | 59 |
Catheter-Associated UTI-Positive Patients According to Sex
A total of 16.67% samples revealed significant leucocyturia (Table 5).
| Number | Proportions, % | |
|---|---|---|
| Leucocyturia ≥ 10/microscopic field | 10 | 16.67 |
| Leucocyturia < 10/microscopic field | 50 | 83.33 |
| Total | 60 | 100 |
Distribution of Samples According to Leucocyturia Status
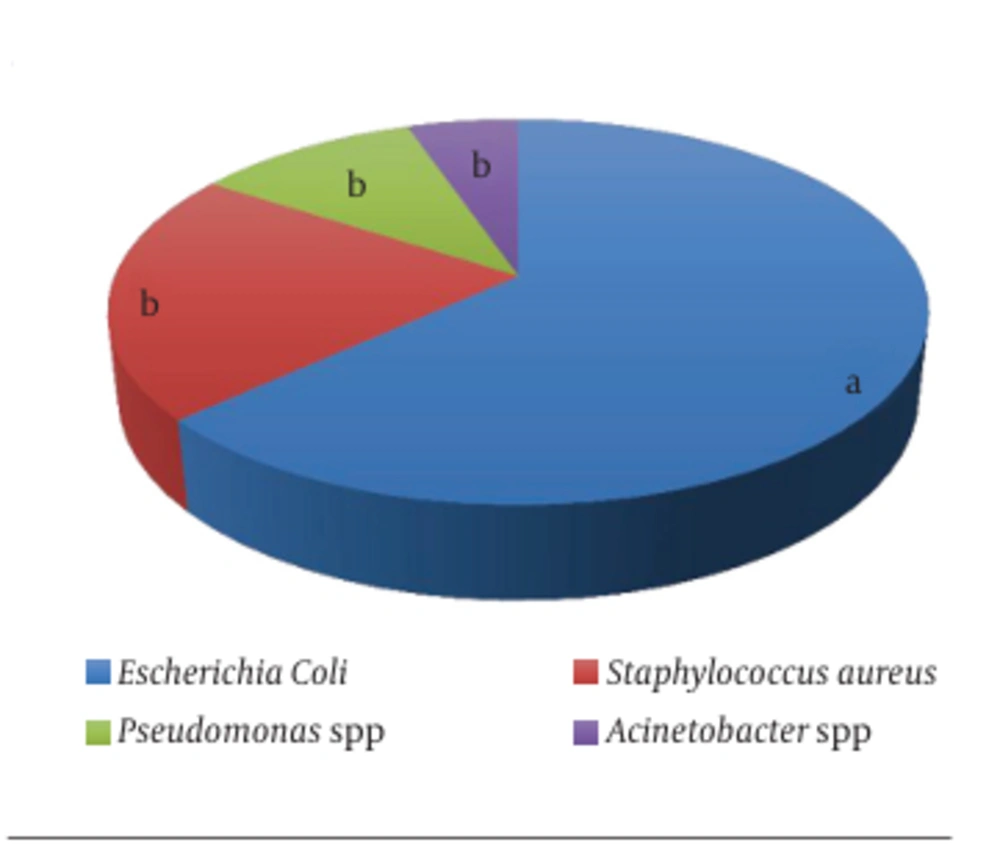
After culturing, the infected urines presented four different bacteria, including Acinetobacter spp. (5%), Pseudomonas spp. (11%), S. aureus (21%), and E. coli (63%). The latter represents the most isolated bacterium among the catheterized patients with significant superiority (P < 0.05) (Figure 6).
Most of the isolated bacteria were from patients in the age group of 50 - 69 years (53%). This proportion is significantly higher than those of the other age groups (P < 0.05) (Table 6).
Out of all the pathologies that required patients’ catheterization and lead to an UTI after operation, urinary retention is the one in which more bacteria were isolated (57.89%, P < 0.05), as shown in Table 7.
Distribution of Bacteria Isolates According to Pathologies That Required Catheterization
All four bacteria species identified were multidrug resistant. The Gram-negative bacilli presented a resistance to cefotaxime and a high sensitivity to ceftriazone, which are commonly prescribed in the hospital (Table 8).
| Bacteria/Phenotypes | Number of Isolates |
|---|---|
| Escherichia coli | |
| AMPI AMXII CROI CTXR CNS CIPS | 2 |
| AMPR AMXR CROS CTXR CNS CIPS | 3 |
| AMPS AMXS CROS CTXR CNS CIPS | 2 |
| AMPR AMXS CROI CTXR CNS CIPS | 2 |
| AMPR AMXR CROS CTXR CNS CIPR | 3 |
| Pseudomonas spp. | |
| AMPR AMXR CROS CTXR CNS CIPS | 2 |
| Acinetobacter spp. | |
| AMPR AMXI CROI CTXR CNS CIPS | 1 |
Resistance Profile of Gram-Negative Bacilli
Gram-positive cocci were resistant to cefoxitine and vancomycin, which are commonly used at the hospital (Table 9).
| Bacteria | Phenotypes | Number of Strains |
|---|---|---|
| Staphylococcus aureus | ES PTS FOXR TES VAR | 4 |
Resistance Profile of Gram-Positive Cocci
5. Discussion
The high prevalence of UTIs revealed by the present study reflects the role of catheters in the contamination of the urinary system. Through the biofilms that are formed around catheters, bacteria can find their way into the urinary tract, leading to infection (4). The role of contamination of the urinary tract by the catheter is revealed in the present study that demonstrates an increase in the proportion of infected patients two days following catheterization (1.6% on Day 1 against 23.33% on Day 3). Furthermore, the hygiene practices observed during the operation being poor, these bacteria can easily colonize the urinary tract from thorough cross-contaminations from various sources, including staff members’ hands, the non-disinfected surfaces of patients, and the environment of the working place. The exacerbating role of the inappropriate conditions of catheterization on the prevalence of UTIs was previously reported by Hooton et al. (11).
Zarb et al. (6) reported a prevalence of 17.2%, lower than 23.33% found in the current study. This difference can be explained by the fact that in Europe, where the lower prevalence was reported, hygiene practices are more rigorous than in developing countries.
The current study reveal that sex is an important risk factor for catheter-associated UTIs. Catheterization in women is affected by higher risks of bacteriuria than in men. This could be explained by anatomical reasons, namely the proximity of the urethral meatus, the vagina, and the anus in women (12).
The fact that the minimal age of UTI-positive patients in this study was 14 years and the maximal 90 years shows that catheter UTIs can affect any category of age in society. However, patients older than 65 seem to have greater exposure to the risk of catheter-associated UTIs. This might be explained by the weak immune status of this cluster of patients due to their age. Moreover, it is reported that, even in the absence of a catheter, being older is one of the factors encouraging the apparition of bacteriuria, although the reasons are not clear (12).
The predominance of E. coli among the Gram-negative bacilli confirms the works of Kocak et al. who showed that this bacterium was the most implicated in catheter-associated UTIs (13).
In addition, the high prevalence of the Gram-negative bacteria like E. coli is in agreement with previous studies (8, 13, 14) This report is related to the physiopathology of UTIs often associated with a strong colonization of the perineum by enteric bacteria of the gastro-intestinal tract or from the environment through catheters. Sekhsokh et al. suggested that specific factors of the pathogenicity of E. coli play an important role, as this bacterium possesses some pili capable of binding to the urinary epithelium and preventing their elimination by urine (8). Moreover, Gram-negative bacterial species that cause catheter-associated UTIs express a number of virulence factors associated with adhesion, motility, biofilm formation, immunoavoidance, and nutrient acquisition as well as factors that cause damage to the host (15). To mitigate the risk of UTIs and other bloodstream infections during catheterization, it might be efficient to use antibiotic impregnated catheters, as recommended by Lorente (16), although such an approach may contribute to increased antibiotic resistance in patients.
S. aureus was the most isolated among the Gram-positive cocci. According to Gaynes and Edwards, the predominance of this bacterium is due to the lipoteichoic acid contained in its cell wall and used as an adherence factor to survive in the urinary tract (17).
E. coli and A. spp. are two unhygienic bacteria, and their isolation in this study shows the presence of one or more contamination sources either from a patient's unwashed body or from the dirty hands (even gloved) of medical personnel, or even from the lack of maintenance of the closed catheter system. There is therefore a necessity to suggest a disinfection of the site before catheterization, an aseptic manipulation of the catheter before its introduction into the urinary tract, and proper maintenance of the whole system of urine compilation. It is important to emphasize that from the moment a catheter is inserted, the risks of bacteriuria and of catheter-associated UTIs increase every day (18).
All bacterial species showed resistance to several antibiotics, particularly those prescribed to catheterized patients. They are multidrug resistant. Therefore, this poses a serious public health problem that needs to be communicated very earlier to health professionals to encourage appropriate measures toward antibiotics prescription to patients in need of anti-biotherapy. Furthermore, this situation suggests that the treatment of UTI should be guided by susceptibility test results when possible or empirical evidence of antibiotics that are known to be sensitive in specific situations. Furthermore, although most of the germs recovered in this study are commensals, it is critical to emphasize their threatening antibiotic sensitivity profile. It has been reported that commensals can serve as reservoirs of multidrug-resistant genes for pathogenic ones to whom they may transmit the resistant genes through various mechanisms (19). Furthermore, the increasing incidence of multi- and extensively drug-resistant Acinetobacter spp. emphasizes the importance of administering an adequate antibiotic strategy and the need for new and effective treatment options, as well as the implementation of strict monitoring of the measures to control all nosocomial infections (20).
The assessment of hygiene practices before patients’ catheterization as well as the maintenance of catheters in the different concerned services revealed that most health workers do not observe good hygiene practices. The practice of patient systematic washing was done in the Surgery department before the catheterization was actually carried out at the operating block and not directly in the Surgery service. It is therefore a routine and mandatory practice that is observed in the Surgery section. Without this specificity, the Department of Surgery would have presented the same situation as the others in which catheterization is achieved without prior patient washing. There is thus a serious ignorance or carelessness of the elementary hygiene rules concerning catheterization in this hospital.
5.1. Conclusion
The present research demonstrated a poor level of respect for hygiene rules during the catheterization of patients in the hospital in Zinvie. The prevalence of catheter-associated UTIs in the study population is 23.33% and caused mostly by E. coli, P. spp, A. spp, and S. aureus. These bacteria showed resistance to several antibiotics. As with all foreign devices introduced in the body, urinary catheters are responsible of a number of nosocomial urinary infections. It is therefore essential to promote rigorous hygiene practices to limit UTIs during all stages of catheterization. It is also important to assess with precision the indications and to limit the duration of catheterization. This is primordial for improving the health in patient populations, particularly in hospital settings.