1. Background
Hepatitis delta virus (HDV) was first discovered by Rizzetto in 1977 in a patient with chronic hepatitis B virus (HBV) infection (1). In 1980, it was shown that HDV was an infectious agent responsible for exacerbation of liver disease in patients with hepatitis (2). The hepatitis D virus (HDV) is a small enveloped virus, with a circular single-stranded negative sense RNA coated with an envelope made up of hepatitis B surface antigen (HBsAg) (3). The virus is considered an animal viroid.
There are eight genotypes (1 to 8) of HDV distributed over different geographic areas. HDV-1 is distributed worldwide, whereas HDV-2 thru 8 are seen locally, closely associated with specific geographic areas. HDV-2 and HDV-4 are found in east Asia (4). HDV-3 had been isolated from the northern area of south America, including the Amazon basin of Brazil, Peru, Colombia, and Venezuela (5), while HDV-5 thru HDV-8 have been identified in individuals from Africa (6). Infection with HDV can occur either via simultaneous infection with HBV (co-infection) or superimposed on chronic hepatitis B or hepatitis B carrier state (super infection). Both super infection and co-infection with HBV results in more severe complications compared to infection with HDV alone (7). The transmission of HDV is similar to hepatitis B, which occurs through blood transfusion, sexual intercourse, and vertically from infected mother to neonates (8). The diagnosis of HDV infection is made following serologic tests for the virus. Every patient who is HBsAg positive should be tested for anti-HDV IgG antibodies, which persist even after the patient has cleared the HDV infection (9). Although active HDV infection has been historically diagnosed by the presence of anti-HDV IgM antibodies, it is now confirmed by the detection of serum HDV RNA with a sensitive real-time PCR assay (9). The epidemiology of HDV has been well studied in developed countries, however, HDV in Africa was found in 20 - 40% of HBsAg carriers (10). Lean information is available on the epidemiology of HDV in Sudan, mainly due to lack of proper laboratory facilities and expertise.
2. Objectives
This study was conducted to determine the seroreactivity and to molecularly detect HDV among hemodialysis patients and blood donors in Khartoum state, Sudan during the period January 2012 to July 2013.
3. Patients and Methods
3.1. Data Collection
Ethical approval for this study was obtained from the Ministry of Health. Only patients who agreed to participate were enrolled in this study and informed consent was obtained regarding the data and the collection of blood samples. The collected data included the name, age, gender, period and place of dialysis, history of jaundice, date of infection with HBV and date of collection.
3.2. Sample Collection
During the period January 2012 - July 2013 a total of 278 blood samples were collected from patients who are chronically infected with HBV. A hundred samples were collected from hemodialysis patients in Khartoum hospitals (Ibin Sina hospital, Dr. Salma center for transplantation and hemodialysis, Omdurman teaching hospital, Alnow hospital, Alamal hospital, and Bshaier teaching hospital). In addition, 178 samples were collected from blood donors at the central Blood Banks in Khartoum, and Omdurman hospitals. Collected blood samples were centrifuged at 5,000 rpm for five minutes to obtain the plasma. The plasma was taken immediately and stored at -80°C until tested.
3.3. Serology
3.3.1. Sandwich Detection ELISA for HBsAg
Commercial ELISA kits were purchased from CTK Biotech, (San Diego, USA). The ELISA procedure was performed according to the manufacturer’s instructions. In brief, 50 μL of the serum was incubated at 37°C for 60 minutes in a 96-well microplate coated with rabbit monoclonal antibodies reactive to HBsAg (anti-HBs). Subsequently, the wells were washed (three times) to remove residual plasma. Fifty μL of anti-HBs conjugated antibody was then added and the wells were incubated at 37°C for 60 minutes. The wells were washed (three times) to eliminate unbound conjugate, 50 μL of enzyme substrate and chromogen was added and the wells were incubated at 37°C for 60 minutes. Fifty μL diluted stop solution (sulfuric acid) was then added to each well, and the plate was read at 450 nm, as indicated by the manufacturer.
3.3.2. ELISA for HDV IgG
The ELISA kit for IgG detection was purchased from Fortress diagnostics limited (United Kingdom). The ELISA procedure was performed according to the manufacturer’s instructions. In brief, 100 μL of the sample diluent and 10 μL plasma were incubated in microplate wells coated with HDV antigen at 37°C for 30 minutes. The wells were washed three times (350 μL washing solution) to remove residual plasma, and 100 μL from enzyme-labeled antibodies to human IgG were then added, and the wells were incubated at 37°C for 30 minutes. After another washing step to eliminate unbound conjugate, 50 μL of chromogen A and 50 μL substrate of chromogen B were added, then the wells were incubated at 37°C for 15 minutes. This was followed by the addition of 50 μL of the stop solution. The plate was read at 450 nm, as indicated by the manufacturer.
3.3.3. ELISA for HDV IgM
The ELISA procedure was conducted according to the manufacturer’s instructions (Fortress Diagnostics Ltd., United Kingdom). In brief, 100 μL of the plasma was incubated at 37°C for 30 minutes in a 96-well microplate coated with anti-HDV IgM antibodies (anti-μchain). Subsequently, the wells were washed three times using a washing buffer to remove residual plasma, and unbound HDV IgM and 100 μL of conjugated HDV Ag was then added to each well, except the blank, and the wells were incubated at 37°C for 30 minutes. After another washing step to eliminate unbound material, 50 μL of enzyme substrate and chromogen was added, and the wells were incubated at 37°C for 15 minutes. This was followed by the addition of 50 μL of stop solution (sulphuric acid). The plate was read at 450 nm, as indicated by the manufacturer.
3.4. Reverse Transcription Polymerase Chain Reactions (RT–PCR)
3.4.1. RNA Extraction
For HDV testing, RNA was extracted from 100 μL of plasma by using a viral RNA extraction kit (Roche Diagnostic, Germany), according to the protocol of the manufacturer.
3.4.2. Complementary DNA Synthesis (cDNA)
Complementary DNA synthesis was conducted by using a cDNA kit (Roche Diagnostic, Germany), according to the protocol of the manufacturer.
3.4.3. Semi-Nested PCR
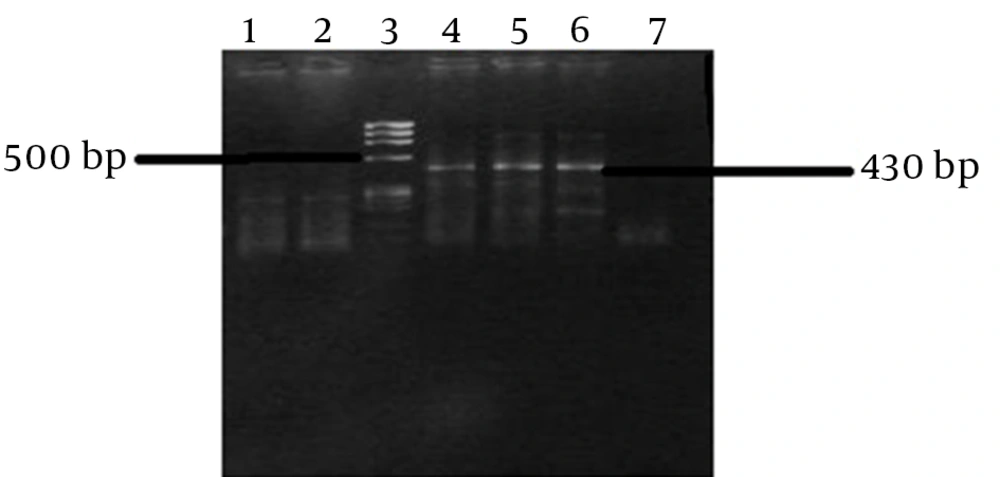
Semi-nested PCR was performed to detect viral cDNA by PCR amplification. The reaction was performed in a total volume of 50 µL in the first PCR reaction, containing 5 µL of cDNA mixed with 20 pmol of each primer (outer primers; forward (900S) 5’- CAT GCC GAC CCG AAG AGG AAA G-3’ and reverse (antisense; 1400 AS) 5’-GAG GGA GCT CCC CCG GCG AAG AG-3’, 5 µL of 2 mM dNTPmix, 2 µL of 25 mM MgCl2, 2.5 U Taq DNA Polymerase (Roche Diagnostics, Germany), 1xbuffer and ddH2O. The amplification was conducted using 35 cycles of PCR reaction (denaturation at 95°C for 30 seconds, annealing at 58 - 54 (touchdown) for 1 minute and extension at 72°C for 45 seconds. The second round of the nested PCR was done with 5 µL the PCR product of the first round, using 20 pmol of each primer (inner primers: forward (900S) 5’- CAT GCC GAC CCG AAG AGG AAA G-3’, and reverse (1280AS) GAA GGA AGG CCC TCG AGA ACA AGA-3’) and applying another touchdown PCR (denaturation at 95°C for 30 seconds, annealing at 60 - 58 (touchdown) and extension at 68°C for 30 seconds. The amplicons were resolved and screened using a 2% agarose gel electrophoresis method. All PCR reactions were performed with appropriate negative and positive controls, which are size band 430 bp to avoid any false negative and positive results.
4. Results
4.1. Detection of HBsAg Among Hemodialysis Patients
Ninety-eight out of 100 (98%) hemodialysis patients, (64 males and 34 females), showed HBV HBsAg in their serum samples (Table 1).
| Gender | Test | |||
|---|---|---|---|---|
| HBsAg | HDV IgM | HDV IgG | HDV RNA | |
| Male | 66 | 64 | 64 | 64 |
| +Ve | 64 (96.9) | 11 (17.2) | 10 (15.6) | 7 (10.9) |
| Female | 34 | 34 | 34 | 34 |
| +Ve | 34 (100) | 2 (5.9) | 6 (11.6) | 6 (11.6) |
| Total | 98 (98) | 13 (13.3) | 16 (16.3) | 13 (13.3) |
Comparison Between ELISA IgG and IgM, and HDV RNA for the Diagnosis of HDV in Plasma Samples Collected From Hemodialysis Patients in Khartoum State (2012)a
4.2. Detection of HBsAg Among Blood Donors
All blood donor patients (100%) (178 males) showed HBsAg in their serum samples (Table 2).
| Gender | Test | ||
|---|---|---|---|
| HBsAg | HDV IgM | HDV IgG | |
| Male | 178 | 178 | 178 |
| +Ve | 178 (100) | 5 (2.8) | 8 (4.5) |
| Female | NA | NA | NA |
| +Ve | NA | NA | NA |
| Total | 98 (98) | 8 (2.8) | 8 (4.5) |
Comparison Between ELISA IgG and IgM for the Diagnosis of HDV in Plasma Samples Collected From Blood Donors in Khartoum STATE (2012)a
4.3. Detection of HDV IgG Antibodies Among Hemodialysis Patient
Sixteen (16.2%) out of 98 hemodialysis patients who were positive for HBsAg showed HDV IgG antibodies in their serum samples. Of these positive patients, 10 were male and 6 were female (Table 1).
4.4. Detection of HDV IgG Antibodies Among Blood Donors
Eight (4.5%) out of 178 male blood donor patients showed HDV IgG antibodies in their serum samples. All of these positive patients were male (Table 2).
4.5. Detection of HDV IgM Antibodies Among Hemodialysis Patients
Thirteen (13.3%) out of 98 hemodialysis patients showed HDV IgM antibodies in their samples. Of these positive patients, 11 were male, while only 2 were female (Table 1).
4.6. Detection of HDV IgM Antibodies Among Blood Donor
Five (2.8%) out of 178 blood donor patients showed HDV IgM antibodies in their samples. All of these positive patients were male (Table 2).
4.7. Semi-Nested RT -PCR Results
HDV RNA was detected in 13/98 (13.3%) samples from hemodialysis patients, of whom 7 (12.2%) were male and 6 (17.6%) were female (Table 1).
5. Discussion
HDV is a subviral agent that can lead to severe acute and chronic forms of liver disease in association with HBV (11).
This study was conducted for serological detection of HDV antibodies and molecular detection of HDV RNA in hemodialysis patients and blood donors in Khartoum State. HBV in Sudan has been shown to cause 22% of fulminant hepatitis cases (12), and 18.5% of Sudanese blood donors have been exposed to the virus (13). In the current study, our findings report high rates of HDV infection associated with HBV in Sudan.
This study revealed that the prevalence of HDV IgG and IgM antibodies among hemodialysis patients was 16.2% (16/98) and 13.2% (13/98), respectively, while in the blood donor group the prevalence of HDV IgG and IgM antibodies was 4.5% (8/178) and 2.8% (5/178), respectively. This result indicated that hemodialysis patients are at higher risk of acquiring HDV infection than those in the blood donor group. Our results also showed that the Salma Center of Renal Transplantation and Hemodialysis had the highest rate of infection, with 20% (4/20), 25% (5/20) and 30% (6/20) HDV IgM, IgG, and HDV RNA, respectively. When compared with other centers of hemodialysis this may indicate the absence of safety precautions during hemodialysis processes.
Furthermore, our results showed the prevalence of HDV IgG and IgM antibodies to be 4.5% (8/178) and 2.8% (5/178), respectively, in the blood donors group. This result was similar to the prevalence of 4.7% (8/170) of HDV IgG, which was found among blood donors in Egypt (12).
The present investigation showed that 13.2% (13/98) of hemodialysis patients were positive for HDV RNA. Of these, only 2.2% (2/98) were positive for HDV IgM antibodies. This indicates that co-infection is the major mode of transmission. In general, males were more frequently infected by HDV, based on the results of ELISA and nested RT-PCR. The nested RT-PCR method, as used in the present study, was shown to be a highly sensitive and specific method for detection of HDV.
These findings highlight the need for establishing rapid, sensitive, and specific diagnostic techniques in Sudan, such as those used in this study, for better management of HDV infection, especially in groups at high risk such as hemodialysis patients. To our knowledge, this is the first attempt to identify HDV in Sudan by using molecular techniques.
The results obtained in this study show the need for wider surveillance and molecular detection at a national level, in order to fully elucidate the true status of HDV infection in Sudan. In this study, the use of nested RT-PCR in the detection of HDV among hemodialysis patients and blood donors was established. This study was conducted to serve as a baseline for future plans aiming to study HDV genotypes in Sudan.
In summary, the incidence and existence of HDV in Sudan was documented through the detection of HDV-specific antibodies (IgG and IgM in serum samples), indicating a high prevalence among hemodialysis patients in Sudan. Moreover, HDV detection using nested RT PCR was established. Generally, these findings are useful for future studies, since there is little information available about HDV infection in Sudan.