1. Background
With the exception of Entamoeba histolytica which may possibly cause brain, lung and liver abscess from a primary focus on the large intestine, pathogenic amoeba species such as Acanthamoeba spp., Naegleria folweri and Balamunthia mandrillaris can cause central nervous system disability called primary amoebic meningoencephalitis (PAM) and granulomatous amoebic encephalitis (GAE) (1, 2). According to some studies, Acanthamoeba species can be classified based on their structure (3). The current study identified 20 species so far (4). Cystic form of these amoebas can survive for many years in the environment, including water, soil, sewage and dust (5-8). Because of the wide distribution, humans can be easily exposed to these amoebas; therefore, they should be separated from skin and nose of healthy people (9). Acanthamoeba cysts are the main cause of amoeba transmission. Cysts can enter the body tissues through water, soil and dust from the outside or from a primary focus in the lung, nose and skin ulcers and cause their virulence. Acanthamoeba species have relatively slow tissue invasion; therefore, granuloma formations are observed in their tissue infection. Chronic granulomatous lesions of pathogenic species of Acanthamoeba are reported in the tissues of brain, kidney, liver, spleen, skin, uterus and prostate (10).
Ocular lesions mainly caused by the invasion of Acanthamoeba spp. occur with corneal ulcers and keratitis (11). If amoebic keratitis not treated, the ulcers lead to stromal puncture, loss of vision and eventually cause blindness. In rare cases, Acanthamoeba spp. could be permeating to retina and cause chorioretinitis (12). Molecular studies showed that most of the amoebic keratitis cases in Iran were A. castellani, A. palestiniensis and A. griffini (13).
In some people, especially in HIV positive patients, Acanthamoeba spp. can include chronic skin ulcers, abscesses or erythematous nodules, especially in the lung. Skin ulcers caused by Acanthamoeba spp. are common in HIV positive patients and may occur alone or in combination with central nervous system (CNS) involvement. Skin ulcers are also reported in patients with amoebic encephalitis and the ones receiving immunosuppressive drugs (7).
Besides, human pathogenic bacteria such as Legionella spp., Cholera spp., Mycobacterium tuberculosis and Helicobacter spp. within Acanthamoeba species are repeatedly reported. Some species of Acanthamoeba were proposed as a reservoir of Legionella spp. in nature (14, 15).
2. Objectives
The current study aimed to determine the soil contamination with Acanthamoeba and Hartmannella spp. in Sari, North of Iran.
3. Patients and Methods
3.1. Study Area
Sari is the provincial capital of Mazandaran, located in North of Iran, between the Northern slopes of the Alborz mountains and the Southern coast of the Caspian sea. Sari is the largest and most populous city of Mazandaran. Human population of Sari is around 196’000. The city is located between the parallels 35° 58 and 36° 50 of the Northern latitude and between 52° 56 and 53° 59 of the Eastern longitude (16).
3.2. Soil Examination
A total of 96 samples were collected from three areas of the city (32 samples per each area). Each specimen contained approximately 200 g of soil from a depth of 2 - 5 cm in an area not exposed to direct sunlight. The soil samples were dried overnight at room temperature. The samples were filtered through 150 μm mesh sieves, yielding approximately 2 g of powdered soil. The powdered sand was moved to a 15 mL test tube, suspended in approximately 10 mL of Tween-80 solution with the concentration of 0.05 and centrifuged at 1500 rpm for 10 minutes.
After discarding the supernatant, the test tube containing the sediments was filled to approximately 1 cm from the top with sucrose solution (specific gravity of 1.200), vortexed, and again centrifuged for 15 minutes at 1500 rpm (17). Finally the tubes were filled to the top with sucrose solution and a cover slip placed on the tube for 30 minutes and then microscopic slides were prepared from flotation materials. The fixed smear was immersed in Giemsa (Merck, Darmstadt, Germany) solution (1: 45 mL of dH2O) in a staining container for 60 minutes. The slide was then rinsed with dH2O (dried at 37°C for 60 minutes) and studied under the light microscope using 100X oil immersion objective.
4. Results
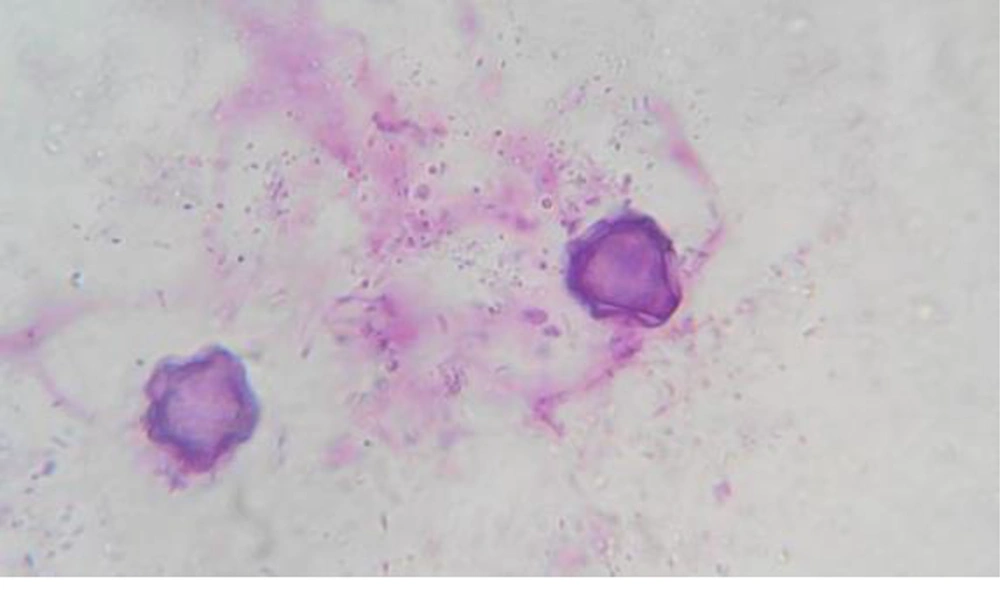
The identification of Acanthamoeba at the genus level in the study was based on distinctive features of double walled cysts (Figure 1). Of the 96 studied samples, from different environmental locations in Sari, 38 (39.6%) Acanthamoeba spp. and 5 (5.2%) Hartmannella spp. were identified. The most contaminated soil contamination was from region 1 (Table 1).
5. Discussion
It was the first study to investigate soil contamination with free-living amoeba in North of Iran. The study isolated Acanthamoeba and Hartmannella spp. from a variety of ecological habitats using flotation methods. Acanthamoeba spp. is isolated from a variety of habitats such as water, seashores, pools, soil, dust, food and hospitals (18). The high rate of incidence in different environmental resources represent a serious warning to the public health, especially in immunocompromised patients (19). To date, northern cities of Iran, especially Sari, attract many tourists annually. The results of the present study revealed that soil resources of this area were contaminated with opportunistic amoebas such as Acanthamoeba spp. and Hartmannella spp. that may lead to severe diseases in high-risk people such as immunocompromised patients. The ability of these protozoans to survive and proliferate in nature, especially water, shows that they can be potentially pathogenic for humans and animals (7). According to the wide dispersion of protozoan, it is expected that many people are exposed to protozoan (20-26); therefore, it seems that 80% of the people have antibodies against protozoa (27).
The 96 processed samples, from three regions in Sari, represent an enormous difference in the incidence between Acanthamoeba spp. and Hartmannella spp. In a study in Spain, fifteen of the 24 samples (62.5 %) were positive for Acanthamoeba spp. based on morphological analysis and PCR results (28). Studies on free-living amoebae and Acanthamoeba spp. in Canary Islands showed that about 40 % of samples from soil and water sources were positive for Acanthamoeba spp. (27). The results of these studies showed that soil contamination with free-living amoebas were similar with those of the present study.
As mentioned, Acanthamoeba species can serve as a vector for many pathogenic microorganisms such as viruses, fungi, protozoa and bacteria (29). Also, studies showed that primary classification of amoebas species were on the basis of morphology and based on the shape, size and amoeba cysts and the three major established groups (3). However, more studies revealed that the morphology of cysts was dependent on the ionic strength of their surrounding environment (30). Therefore, only molecular methods allow reliable differentiation of the Acanthamoeba species (8). To date, 18 Acanthamoeba genotypes are identified (31). A number of these genotypes such as T3, T11 and even T5 are noted to result in human clinical manifestations (32).
Ultimately, in order to reduce the health risk and avoid infections especially in immunocompromised patients from pathogenic strains of free-living amoebae, active surveillance to identify and control the presence of potentially hazardous Acanthamoeba species in other resources in these areas seem necessary. Therefore, the study can serve as a preliminary study in this area for further research to determine the genotypes of strains providing Acanthamoeba spp. and Hartmannella spp.