1. Background
Anthrax is caused by a Gram-negative, nonmotile, rod-shaped bacterium, known as Bacillus anthracis. There are uncertainties about prophylactic approaches against anthrax, such as multiple booster doses, toxicity, use of adjuvants, and stability. The protective antigen of B. anthracis is the principal component of all approved human vaccines against anthrax. However, this protective antigen has problems, such as stability and selection of suitable adjuvants. Overall, encapsulation of protective antigen in biodegradable polymers has not been successful, so far (1).
Protective antigen domain 4 (PAD4) is responsible for binding with the host anthrax toxin receptors, encoded by tumor endothelial marker-8 and capillary morphogenesis gene-2 receptors (2). The interaction of PAD4 with the host cell and monoclonal antibody has been studied in great detail (3-6). PAD4 has been evaluated in various vaccine formulations, such as plant-based (7) and alfalfa mosaic virus-mediated expression systems (8). Moreover, it has been applied with rabies virus glycoprotein as a carrier protein (9), calreticulin as an adjuvant (10), and lethal factor as a fusion protein (11).
The crystal structure of isolated PAD4 has a structural conformation similar to PAD4 in native protective antigen molecules (12). PAD4 can maintain its structural integrity and bind with host cell receptors at an acidic pH (12). This property of PAD4 can be an advantage for poly (lactide-co-glycolide) (PLGA)-based encapsulation, as an acidic pH environment is induced during PLGA degradation (13). This environment can cause protein aggregation, oxidation, deamidation, and acylation (14, 15). Also, PAD4 can be produced by recombinant protein expression, thus providing a safe and defined immunogen (16).
In our previous study, we showed that recombinant PAD4 can be successfully encapsulated in a PLGA-based particulate system (17, 18). However, there is an intricate relationship between the parameters of PLGA particulate process and immunogenicity. Surface porosity reduces the antigen content in PLGA particles (19). Overall, particle size has been considered as a major influencing parameter for immunogenicity. However, there are conflicting reports on the correlation of particle size with the resultant immune response (20). The polymer composition can change the hydrophobicity of polymer matrices (21); antigen dose has been also reported to influence the desired immune response (22).
With this background in mind, in this study, the process parameters and their effects on the elicited immunogenicity were studied. Three adaptations of w/o/w method were used with 2 compositional variants of PLGA. The particles were evaluated to achieve an optimized immunoglobulin G (IgG) response. The hydrophobic particulates with the mean diameter of ~ 3 µm elicited the highest IgG titer among all the evaluated particle variants.
2. Methods
2.1. Purification of Recombinant PAD4
PAD4 was purified via Ni-NTA denaturing affinity chromatography with urea denaturation lysis on column renaturation, as described in the literature (17, 18). The bacterial pellets, used for the purification steps, were collected from a 4 L Escherichia coli culture; therefore, the antigen used for particle formulations was from the same batch. Also, 250 mM NaCl was used in the final antigen stock buffer to prevent protein aggregation (12).
2.2. Formulation of PLGA Nanoparticles/Microparticles
Two polymer variants of PLGA (Sigma-Aldrich; P2191-5G lot #051M1298V and 430471 lot #MKBG1113V) were used in this study (Table 1). The polymers were of similar molecular weight and inherent viscosity, but of different monomer compositions. Also, polymer end-groups were ester-terminated.
| Variants | PLGA 1 | PLGA 2 |
|---|---|---|
| Composition | 50:50 (52:48) | 85:15 |
| Molecular weight | 40000 - 75000 | 50000 - 75000 |
| Inherent viscosity, dL/g | 0.55 - 0.75 (0.61) | 0.55 - 0.75 (0.65) |
| Ester-terminated | Yes | Yes |
| Form | Amorphous | Amorphous |
| Transition temperature, °C | 45 - 50 | 45 - 50 |
The PLGA polymer variants used in this study. Values within the brackets provide the lot-specific values. Table 1 shows that polymer variants used in this study only varied with respect to lactide content.
PLGA particulates were prepared via 3 methodological adaptations of w/o/w solvent evaporation method. In the first method, 200 mg of PLGA was dissolved in 4 mL of dichloromethane, and a 100 µL aliquot of concentrated recombinant PAD4 (10 mg/mL) was used as the internal aqueous phase. W/O emulsion was prepared using 2 mm of stepped microtip at 35% amplitude for 60 seconds (750 W, Sonic Vibra-Cell™ sonicator).
An aqueous solution of 1% polyvinyl alcohol (PVA) was used as the external aqueous phase. The w/o/w emulsion was prepared, using 6 mm stepped tip at 30% amplitude for 110 seconds. The emulsion was stirred for 6 hours for the evaporation of dichloromethane. Particles were harvested by centrifugation at 15,000 g for 15 minutes and washed 3 times with sterile deionized water.
Subsequently, the particles were suspended in 5 mL of sterile deionized water and frozen in liquid nitrogen to avoid phase separation. Particles were kept at -80°C for 1 hour and then lyophilized at -54°C and 0.003 mBar for 18 hours. The PLGA particles, prepared with the first method, were in the nanometer size range. Particles prepared from PLGA (50:50) and PLGA (85:15) were termed as PAD4-NP1 and PAD4-NP2, respectively.
In the second method, the process parameters remained similar to method 1, except for the preparation of w/o/w emulsion via homogenization with an IKA homogenizer probe (S10N-10G) at speed of 2 for 10 minutes. The effect of NaCl on particle porosity was also explored by varying the concentration of NaCl in the external aqueous phase. The particles were harvested by centrifugation at 1500 g for 5 minutes. PLGA particles, prepared by method 2, were in the micrometer size range. The particles prepared with PLGA (50:50) and PLGA (85:15) were termed as PAD4-MP1 and PAD4-MP2, respectively.
In the third method, 352 mg of PLGA was dissolved in 800 µL of dichloromethane. The concentrated recombinant PAD4 (10 mg/mL) was used in 100 µL aliquot as the internal aqueous phase. W/O emulsion was prepared via homogenization, using the IKA 10N-5G probe at maximum speed for 30 seconds. Also, 800 µL of 1% PVA was used as the external aqueous phase. The w/o/w emulsion was prepared via homogenization, using the IKA homogenizer probe (S10N-5G) at maximum speed for 30 seconds.
The W/0/W emulsion was transferred to 8 mL of 0.5% PVA and stirred for 2 hours to evaporate dichloromethane. The particles were harvested by centrifugation at 1500 g for 5 minutes. PLGA particles, prepared by method 3, were in the micrometer size range. The particles prepared using PLGA (50:50) and PLGA (85:15) were termed as PAD4-MP3 and PAD4-MP4, respectively.
2.3. Characterization of Particle Morphology
The morphological characteristics of PLGA particulates were evaluated, using a scanning electron microscope (Zeiss EVO40, Carl Zeiss, Thronwood, NY, USA) and a transmission electron microscope (JEM 2100F, Jeol Ltd., Tokyo, Japan), as described in the literature (Manish et al. 2013; Manish et al. 2016).
2.4. Determination of Particle Size
Particle size was measured by light scattering and polarization intensity differential scattering (PIDS) as a specific measure (LSTM 13 320 SW, Beckman Coulter Inc). Also, complete Mie theory was used as an optical model.
2.5. Determination of Antigen Content
The antigen content of PAD4 in PLGA particulate systems was evaluated, using micro-bicinchoninic acid assay (micro-BCA assay). Acetonitrile was used to dissolve PLGA and precipitate PAD4. The precipitated PAD4 was dissolved in 1% sodium dodecyl sulfate (SDS), as described in the literature (17, 18).
2.6. Ethical Considerations
Mice experiments were carried out under the regulations of the Institutional Animal Ethics Committee, Jawaharlal Nehru University, New Delhi, India. Inbred BALB/c mice were procured from the national institute of nutrition (NIN), Hyderabad, India. The mice were housed in individually ventilated animal cages in a pathogen-free, controlled environment.
2.7. Evaluation of IgG Response
The anti-PAD4 IgG response was evaluated, using enzyme-linked immunosorbent assay (ELISA), as described in the literature (Manish et al. 2013). Briefly, the wells were coated overnight with 100 µL of PAD4 (5 mg/mL). Individual serum samples were serially diluted in phosphate-buffered saline (PBS), assayed in triplicate alongside the serum from unimmunized mice, and incubated for 2 hours.
Horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (sc2005, Santa Cruz Biotechnology) was used as the secondary antibody. TMB substrate reagent (BD OptEIATM) was added as a colorimetric substrate and incubated for 30 minutes. The cutoff point was calculated for every serial dilution with a confidence interval of 99% for increased specificity (Frey et al. 1998). A higher confidence interval could reduce sensitivity and decrease the risk of overtitration. The endpoint titer was calculated as the reciprocal value of the highest dilution, having an absorbance above the cutoff point.
2.8. Statistical Analysis
The statistical significance of particle size, antigen content, and immune response was measured by one-way ANOVA and Turkey’s multiple-comparison test.
3. Results
PAD4 was purified via Ni-NTA affinity chromatography. The w/o/w solvent evaporation method was employed for the preparation of PLGA particles. In the w/o/w solvent evaporation method, the antigen, which needs to be encapsulated, is dissolved in the internal aqueous phase. Therefore, to achieve a higher antigen content in PLGA particles, a higher concentration of antigen in the internal aqueous phase is required. In this study, we used PAD4 at a concentration of 10 mg/mL. However, recombinant proteins frequently aggregate/precipitate at higher concentrations (23). NaCl has been reported to reduce the aggregation of PAD4 (12). Consequently, 250 mM NaCl was used in the final PAD4 stock buffer to maintain the uniformity of the solution.
3.1. Sodium Chloride in the External Aqueous Phase Was Required to Achieve Nonporous and Spherical Microparticles
PAD4-NP1 and PAD4-NP2 were prepared using method 1, and the size of particles was in the nanometer range. In method 1, the ratio of organic phase to the internal aqueous phase was 40:1, whereas the dispersed-to-continuous phase ratio was 1:3. First, emulsion was prepared via sonication at 35% amplitude for 60 seconds. The second emulsion was prepared via sonication at 30% amplitude for 110 seconds. The formulated nanoparticles were nonporous and spherical (Figure 1).
A, Transmission electron micrograph of PAD4-NP2. This figure shows that PAD4-NP2s are in the nanometer size range and spherical with a smooth surface. B, Scanning electron micrograph of PAD4-MP1. This figure shows that PAD4-MP1s are in a micrometer size range and have a porous morphology. C, Scanning electron micrograph of PAD4-MP2. This figure shows that PAD4-MP2s are in a micrometer size range and have a porous morphology. D, Scanning electron micrograph of PAD4-MP1. PAD4-MP1 was prepared using 1% NaCl in the external aqueous phase. This figure shows reduction in the porosity of PAD4-MP1. E, Scanning electron micrograph of PAD4-MP1. PAD4-MP1 was prepared using 2% NaCl in the external aqueous phase. This figure shows reduction in PAD4-MP1 porosity. F, Scanning electron micrograph of PAD4-MP1. PAD4-MP1 was prepared using 3% NaCl in the external aqueous phase. This figure shows that PAD4-MP1s are in the micrometer size range and spherical, with a smooth surface. G, Scanning electron micrograph of PAD4-MP3. This figure shows that PAD4-MP3s are in a micrometer size range and have a porous morphology. H, Transmission electron micrograph of PAD4-MP3. PAD4-MP3 was prepared using 1% NaCl in the external aqueous phase. This figure shows that PAD4-MP3 is in a micrometer size range and spherical with a smooth surface.
PAD4MP-1 and PAD4-MP2 were prepared using method 2, and the size of particles was in the micrometer range. In this method, the ratio of organic phase to internal aqueous phase was 40:1, whereas dispersed-to-continuous phase ratio was 1:3. The first emulsion was prepared by sonication at 35% amplitude for 60 seconds, while the second emulsion was prepared via homogenization for 10 minutes. The formulated microspheres showed a porous morphology (Figure 1B and Figure 1C and supplementary file, appendix 1).
In the first and second methods, similar polymer concentrations, organic solvents, internal aqueous phases, and external aqueous phases were used. Particles in the nanometer range were of smooth surface characteristics, whereas particles in the micrometer range had porous surface features. Formation of pores on the particle surface is reported as a complex phenomenon (Giteau et al. 2008); however, in our study, the porosity of PLGA particles was associated with increased particle size (comparison of Figure 1A with Figure 1B and Figure 1C and supplementary file, appendix 1). The porosity of particle surface was associated with both hydrophilic (PLGA 50:50) and hydrophobic (PLGA 85:15) polymers (Figure 1B and Figure 1C).
NaCl in the external aqueous phase has been reported to achieve a nonporous particle formulation (De Rosa et al. 2003). Therefore, NaCl concentration gradient in the external aqueous phase was employed for PAD4-MP1 and PAD4-MP2 formulations. PAD4-MP1 was prepared with 1% NaCl (Figure 1D), 2% NaCl (Figure 1E), and 3% NaCl (Figure 1F). It was observed that the increased NaCl concentration reduced the surface porosity of PAD4-MP1 (Figure 1D and E E and F).F).
NaCl gradient in the external aqueous phase was also employed for PAD4-MP2. PAD4-MP2 was formulated in 1% NaCl (supplementary file, appendix 2), 2% NaCl (supplementary file, appendix 3), and 3% NaCl (supplementary file, appendix 4). It was observed that the increased NaCl concentration reduced the surface porosity of PAD4-MP2 (supplementary file, appendixes 2, 3, and 4).
It was speculated that pore formation could be a result of low PLGA concentration or sonication force used for the formulation of PAD4-MP1 and PAD4-MP2. Therefore, high concentration of PLGA and mechanical force in form of homogenization were used for the formulation of PAD4-MP3 and PAD4-MP4. However, surface porosity was observed in PAD4-MP3 (Figure 1G and supplementary file, appendix 5) and PAD4-MP4 (supplementary file, appendix 6). Even in method 3, surface porosity was associated with both hydrophilic and hydrophobic polymers (Figure 1G and supplementary file, appendix 6). The addition of NaCl to the external aqueous phase reduced the porosity of PAD4-MP3 (Figure 1H) and PAD4-MP4 (supplementary file, appendix 7).
3.2. The Explored Methods for the Preparation of PLGA Particulates Produced Particles of Single-Peak Population with a Nanometer or Micrometer Size Range
Particles PAD4-NP1, PAD4-NP2, PAD4-MP1 (prepared in 3% NaCl), PAD4-MP2 (prepared in 3% NaCl), PAD4-MP3 (prepared in 1% NaCl), and PAD4-MP4 (prepared in 2% NaCl) were evaluated for population size characteristics. Particle size has been considered as a major influencing factor for the desired immune response; therefore, the explored methods were examined for particle population distribution.
Method 1 produced particles in the nanometer range with a single-peak particle population. The particle diameter varied from approximately 50 nm to 500 nm. Method 2 also produced particles with a single-peak particle population; however, the particles were in the micrometer range. Particle diameter varied from approximately 1.5 µm to 10 µm. The particle populations, formulated by methods 1 and 2, showed distinct distributions with no overlap (Figure 2).
Method 3 also produced particles with a single-peak particle population. The particle diameter varied from approximately 3.5 µm to 30 µm. There was no overlap between the particle populations formulated by methods 1 and 3. However, the particle population formulated by methods 2 and 3 overlapped, although the overlap was less than 20% (Figure 2).
The graph was drawn by LS 13320 software (Beckman Coulter, Inc). This figure shows that particle populations, formulated by methods 1 and 2, do not overlap. The particle populations, formulated by methods 1 and 3, also do not overlap. Particle populations, formulated by methods 2 and 3, overlap, although it is less than 20%.
The mean diameter of PAD4-NP1 was 0.230 µm, whereas the mean diameter of PAD4-NP2 was 0.193 µm (Table 2). There was no significant difference between the polymer variants with a nanometer size range. Also, the mean diameter of PAD4-MP1 was 3.66 µm, whereas the mean diameter of PAD4-MP2 was 2.96 µm. In methods 1 and 2, similar polymer concentrations, organic solvents, internal aqueous phases, and external aqueous phases were used.
| PLGA Particulate systems | Arithmetic Mean Diameter (Mean ± SD of 3 Independent Measurements), µm |
|---|---|
| PAD4NP1 (50:50) | 0.230 ± 0.084 |
| PAD4NP2 (85:15) | 0.193 ± 0.068 |
| PAD4MP1 (50:50) | 3.66 ± 1.45 |
| PAD4MP2 (85:15) | 2.96 ± 1.43 |
| PAD4MP3 (50:50) | 7.21 ± 3.16 |
| PAD4MP4 (85:15) | 6.86 ± 5.49 |
Size of PLGA particulates. The particle size was measured by light scattering and polarization intensity differential scattering (PIDS) as a specific measure (LSTM 13 320 SW, Beckman Coulter, Inc). Complete Mie theory was used as the optical model. The results are presented as mean ± standard deviation (SD) of 3 independent samples.
The only difference between methods 1 and 2 was the applied mechanical force. Therefore, the mean diameter of particles varied mainly due to the applied mechanical forces. The difference between the mean diameters of PAD4-MP1 and PAD4-MP2 was not significant; consequently, PLGA polymer variants did not influence the particle size. The mean diameter of PAD4-MP3 was 7.21 µm, whereas the mean diameter of PAD4-MP4 was 6.86 µm. The effect of polymer variant on particle size was not observed in method 3.
Methods 1 and 2 showed a narrow-peak particle population distribution, whereas in method 3, the particle diameter varied from 3.5 to 30 µm. In method 3, the polymer concentration was increased to 440 mg/mL, whereas in methods 1 and 2, the polymer concentration was 50 mg/mL. In method 3, volume of the organic phase was 800 µL, which is 5 times less than the volume of the organic phase, used in methods 1 and 2. These factors constitutively enhanced the viscosity of the organic phase in method 3.
A viscous solution was needed to increase the size of particle formulation. However, viscous solutions are difficult to emulsify, which can contribute to the wide particle population distribution observed in method 3, although maximum homogenization speed was employed. As our objective was to delineate the effect of particle parameters on PAD4 immunogenicity, we mainly concentrated on particles with a mean diameter in nanometer or up to 10 µm. It has been reported that particles greater than 10 µm induce a low humoral antibody response (19). However, the influence of particle size on immunogenicity was not clearly elucidated from nanometer to 10-µm particle populations (20). As the particle sizes were in the desired range, we evaluated the antigen content in PLGA particulates.
3.3. Antigen Content in PLGA Particulates Varied with PLGA Composition and Applied Methods
The antigen was extracted from PLGA particulates and measured using micro-BCA assay. The antigen content in 10 mg of PAD4-NP1 was 50 µg, whereas the antigen content in 10 mg of PAD4-NP2 was 38.22 µg (Table 3). Therefore, a significant change in antigen encapsulation was observed by varying the PLGA composition. Hydrophilic polymer showed a greater antigen encapsulation than hydrophobic polymer in method 1.
| PLGA Particulate Systems | Encapsulated Antigen per 10 mg of Lyophilized PLGA Particulates, µg |
|---|---|
| PAD4-NP1 (50:50) | 50 ± 0.68 |
| PAD4-NP2 (85:15) | 38.22 ± 0.7 |
| PAD4-MP1 (50:50) | 37.18 ± 0.65 |
| PAD4-MP2 (85:15) | 58.31 ± 0.86 |
| PAD4-MP3 (50:50) | 33.87 ± 0.96 |
| PAD4-MP4 (85:15) | 30.68 ± 0.76 |
The encapsulated antigens in PLGA particulate systems. The antigen content in lyophilized PLGA particulates was measured using micro-BCA assay after antigen extraction with acetonitrile and solubilization of antigen precipitate with 1% SDS. The results are presented as mean ± standard deviation (SD).
The antigen content in 10 mg of PAD4-MP1 was 37.18 µg, whereas the antigen content in 10 mg of PAD4-MP2 was 58.31 µg (Table 3). Even in method 2, a significant change in antigen encapsulation was observed by varying the PLGA composition. However, in method 2, the hydrophobic polymer showed a greater antigen encapsulation than the hydrophilic polymer. Therefore, the polymer variants showed a differential behavior with antigen encapsulation, which depended on particle size.
The antigen content in 10 mg of PAD4-MP3 was 33.87 µg, whereas the antigen content in 10 mg of PAD4-MP4 was 30.68 µg (Table 3). The antigen content in particles formulated by method 3 was less than that of methods 1 and 2. In method 3, a higher concentration of PLGA was used, i.e., 352 mg of PLGA was dissolved in 800 µL of dichloromethane. Consequently, a reduction was observed in antigen content per 10 mg of dried PLGA particulates. In this study, antigen content was evaluated as encapsulated antigen per 10 mg of dried PLGA particulates. As PLGA particulates are known to act as an antigen depot and a controlled delivery system, the antigen encapsulated in the particle can be a better correlate for immunogenicity than the antigen encapsulated in the overall formulation process (22).
3.4. PAD4-MP2 Elicited the Highest anti-PAD4 IgG Titer Among the Evaluated PLGA Particulates
The immunization study was carried out on inbred BALB/c mice; the number of mice in each group was 5. The mice were immunized with 100 µg of encapsulated antigen in form of PLGA particulates. The control group received 50 µg of PAD4 alone. The anti-PAD4 IgG response was measured, using an adjuvant, single-dose immunization schedule. A group of mice was also immunized with blank PLGA particles; however, no anti-PAD4 IgG response was detected in this group.
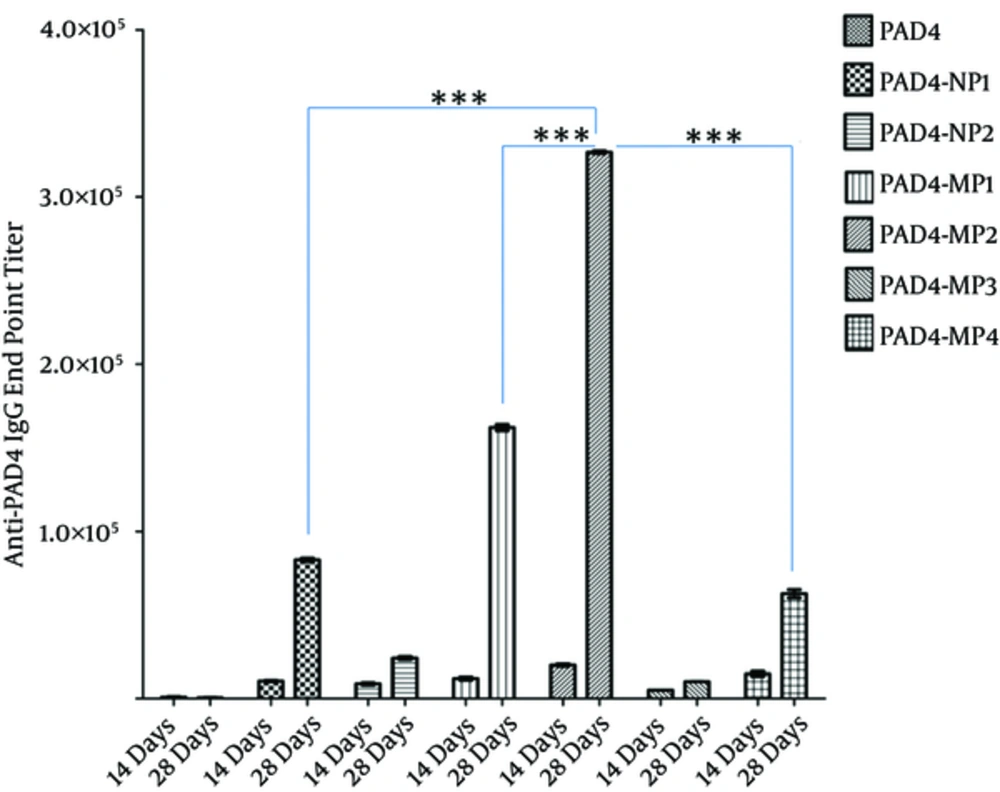
The mice, only immunized with PAD4, showed mean anti-PAD4 IgG titers of 853.3 and 533.3 on days 14 and 28, respectively. The mean IgG titers in PAD4-NP1-immunized mice were 10,500 and 82,973 on days 14 and 28, respectively. Also, the mean IgG titers in PAD4-NP2-immunized mice were 8833 and 24,000 on days 14 and 28, respectively. The mean IgG titer in PAD4-MP1-immunized mice was 36,000 and 485,840 on days 14 and days 28, respectively.
Moreover, the mean IgG titers in PAD4-MP2-immunized mice were 59,480 and 980,680 on days 14 and 28, respectively. The mean IgG titers in PAD4-MP3-immunized mice were 15,220 and 30,580 on days 14 and 28, respectively. Also, the mean IgG titers in PAD4-MP4 immunized mice were 44,000 and 188,000 on days 14 and 28, respectively (Figure 3).
The sera from immunized mice (n = 5) were collected on days 14 and 28 post-immunization. PAD4-MP2 elicited the highest level of IgG titer among the evaluated PLGA particulates. Antibody titers (Y-axis) are presented on a linear scale. Error bars indicate ± standard error (SE) of 3 experiments. ***denotes a statistically significant change (P < 0.001), as evaluated by one-way ANOVA and Tukey’s multiple-comparison test.
PAD4 (in the absence of adjuvants with single-dose immunization) did not elicit a significant IgG titer. However, PAD4-PLGA particulates elicited a robust anti-PAD4 IgG response. PAD4-NP2 induced the lowest antibody titer among the evaluated particulates. IgG titer in PAD4-NP2-immunized mice was approximately 40 times higher than the titer elicited in mice immunized with only PAD4. Therefore, enhancement of IgG titer with particulate formulation was clearly observed, compared to antigen alone.
Among PLGA particulates, PAD4-MP2 elicited the greatest IgG response. Particles with the mean diameter of ~3 µm (i.e., PAD4-MP1 and PAD4-MP2) elicited the highest antibody titer, compared to particles with the mean diameters of ~ 0.2 µm (i.e., PAD4-NP1 and PAD4-NP2) and 7 µm (ie, PAD4-MP3 and PAD4-MP4). The hydrophilic polymers elicited the highest IgG titer in nanometer size range particles. In the micrometer size range, particles from the hydrophobic polymer elicited the greatest IgG response. The antigen content in particles was positively correlated with the immune response (Figure 3).
4. Discussion
Development of recombinant proteins leads to the identification of numerous recombinant antigens. However, use of antigens as vaccine candidates is limited by issues, such as fragile structure, proteolytic degradation, and booster doses. Previously, we showed that PAD4 could be successfully encapsulated in PLGA-based particulate systems. However, the effect of process parameters on particle characteristics and elicitation of IgG response was not clear. This study focused on the evaluation of PLGA-PAD4 variants for enhanced IgG response.
Addition of salt is often required to stabilize the protein solution (24). Particle porosity has been attributed to high osmotic pressure inside the internal aqueous phase (25). Addition of NaCl to the external phase seems to reduce the osmotic pressure difference between the internal and external aqueous phases (25, 26). Particles with a porous surface lead to an enhanced initial burst release. Initial burst release has been considered as a major drawback of PLGA-based particulate vaccine formulations (27).
The pores on the particle surface can reduce the antigen encapsulation (19). Particle porosity is also known to influence the antigen release rate (28). The biodegradation of porous PLGA particulates is reported to be faster than nonporous PLGA particulates (29). Therefore, porous particles can have pharmaceutical disadvantages due to substantial antigen release in the initial burst phase. Loss of antigen in porous particle formulations reduces its economical viability. Furthermore, great initial antigen release from porous particles reduces the efficacy of PLGA particulates to provide a long-term antigen release system (30). Therefore, we obtained nonporous PLGA particulates for the evaluation of particle characteristics and the associated immune response.
We observed the influence of PLGA composition variants on the antigen content of the formulated particles. In the study of rifampicin-loaded microsphere (size, 2 µm), the loading efficiency decreased in PLGA polymers with a high molecular weight. The reason could be the interaction of terminal carboxyl groups of PLGA with rifampicin. At higher molecular weights, the PLGA composition tends to influence the loading efficiency, while at lower molecular weights, the difference in PLGA composition does not influence the loading efficiency (31). As we evaluated the PLGA composition variants of high molecular weight, differential encapsulation of PAD4 in PLGA particles could be explained. It has been also reported that PLGA composition of 75:25 has a higher loading efficiency than PLGA composition of 50:50 for hepatitis B surface antigen-loaded particles with a size range of 2 - 4 µm (32).
In the present study, the greatest IgG response was observed after immunization with PLGA polymer (85:15; mean diameter ~ 3 µm). In the study of PLGA encapsulated hepatitis B surface antigen, the hydrophobic polymer (PLGA, 75:25) elicited a better IgG response than the hydrophilic polymer (PLGA, 50:50) (32). The chemical composition of polymers does not influence the extent of phagocytosis by antigen-presenting cells (33). However, antigen release from particulate systems is dependent on the chemical composition of polymer (21). The antigen release profile is also dependent on the antigen content of particles (21).
PLGA particles with a diameter smaller than 10 µm are reported to be more immunogenic than particles with a diameter more than 10 µm (19). This phenomenon can be attributed to the phagocytic activity of antigen-presenting cells. In the study of phagocytosis of PLGA microparticles, loaded with anti-tuberculosis rifampicin (diameter, 1 - 10 µm) by alveolar macrophages, a considerable proportion of macrophages were not able to phagocytose 1-µm particles. The phagocytosis was greater with 1 and 3 µm particles than 6 and 10 µm particles (34).
In the study of phagocytosis of albumin-loaded polystyrene particles by alveolar macrophages, internalization of particles with a diameter of 500 nm was 10 times more than particles with a diameter of 1 µm and 100 times higher than 2 - 3 µm particles (35). Therefore, differential interaction of particles with antigen-presenting cells can be speculated, based on the size of PLGA particulates. The polymer degradation rate of particles is not influenced by size. In the study of BSA-encapsulated PLGA particles with sizes of 0.1, 1, and 10 µm, the polymer degradation rate did not significantly vary (36).
With respect to the discussed issues, it can be inferred that PLGA composition variants modulate the immune response via differential antigen release, whereas particle size variants modulate the immune response via differential phagocytosis. Therefore, in the present study, we evaluated the composition and size variants to achieve optimum immune response.
In the present study, we observed a greater elicitation of IgG with microparticles, compared to nanoparticles. The higher elicitation of IgG with microparticles has been also reported for PLGA-encapsulated hepatitis B surface antigen (37). Also, an increase in IgG response has been observed by increasing the size of particles. By the comparison of 200, 500, and 1000 nm PLGA particles, encapsulating bovine serum albumin, 1000 nm particles elicited the highest IgG titer (38).
The immune response obtained in 500-nm particles was similar to 200-nm particles in the evaluated immunization routes, i.e., subcutaneous, oral, and intranasal. The IgG1/IgG2a ratio did not change during the evaluation of particulate antigens, which suggests that particles with these size ranges follow the same antigen presentation pathway (38). However, an optimum size range is required for the greater elicitation of IgG response, as in the study of hepatitis B surface antigen, 5-µm particles showed greater immunogenicity than 12-µm particles (19).
4.1. Conclusion
We evaluated 6 variants of PLGA-encapsulated PAD4. The results could help achieve nonporous PLGA-PAD4 variants with a robust IgG response and design novel vaccines against anthrax. However, antigen release and phagocytosis also depend on the surface charge of particles; this factor was not considered in the current study. In the formulation parameters, only 3 methods were evaluated. However, statistically validated experimental designs, such as the Plackett-Burman design (39), are needed to delineate the effects of formulation parameters at low resolutions.