1. Background
A novel virus called coronavirus (SARS-CoV-2) is responsible for the coronavirus disease 2019 (COVID-19) pandemic, which kills millions of people worldwide (1). In addition to causing illness, viral infections can lead to secondary bacterial infections, which can be even more dangerous and destructive than initial viral infections (2). Patients in hospitals, particularly those in intensive care units (ICUs), typically get secondary lung bacterial infections (although secondary fungal or viral infections are less prevalent) (3). Research indicates that the COVID-19 epidemic has increased the frequency of healthcare-associated infections (HAI) (4). Acute COVID-19 patients are more susceptible to HAI, particularly bloodstream infections and pneumonia brought on by multidrug-resistant organisms (MDRs). The HAIs lengthen hospital stays and the need for mechanical ventilation, and they raise the chance of patient death in cases involving septic shock (5, 6).
Antibiotics’ involvement in treating COVID-19 patients is still not well understood, and there are no established standards for the kind, quantity, or duration of antibiotic administration. Although numerous studies have demonstrated the unrestricted use of broad-spectrum antibiotics for the treatment of severe cases of COVID-19, it is still unclear how antibiotic therapy affects patients who are hospitalized in critical care units and whether it has any positive effects (7). Therefore, for people who have been proven to have bacterial infections as well as those who exhibit symptoms and indications suggestive of bacterial infections, prudent use of antibiotics is advised (8). The four antibiotics most frequently provided to the patients under study were azithromycin, vancomycin, meropenem, and the third and fourth generations of cephalosporins (such as ceftriaxone and cefepime). The preceding four medications are more significant than other medications like metronidazole, linezolid, cefazolin, cefixime, and clindamycin (9).
A major public health issue linked to the improper use of antibiotics worldwide is antimicrobial resistance (AMR). The misuse of antibiotic prescriptions is the primary cause of AMR (10). According to recent research on the use of antibiotics in human contexts, the COVID-19 pandemic may have made antibiotic overuse and inappropriate prescribing worse while also accelerating the global spread of antibiotic resistance (11, 12). Mycoplasma pneumonia, Pseudomonas aeruginosa, and Haemophilus influenza were the microorganisms most commonly recovered from induced sputum and/or bronchial cavities, according to some preliminary findings on co-infections in COVID-19 patients (13).
Antibiotics were not advised to treat patients with mild or moderate COVID-19 in any of the nine iterations of the Iranian Ministry of Health’s COVID-19 Guidelines unless there was a clinical suspicion or laboratory confirmation of bacterial infection (14-19). It was mentioned that prescribing antibiotics for COVID-19 inpatients is not recommended, and emphasis was made on the necessity of adhering to antimicrobial stewardship standards for all antibiotic prescriptions (20). It is still essential to comprehend antibiotic use trends in the early phases of the COVID-19 pandemic in order to improve antimicrobial stewardship tactics and guide future outbreak responses. Widespread empirical antibiotic prescribing resulted from the combination of the urgency of early pandemic care and the lack of diagnostic certainty. By examining this time frame, healthcare systems can find important gaps, evaluate compliance with new recommendations, and create focused actions to lower AMR in the event of future health emergencies. The need for examining first-wave prescribing patterns in order to develop more robust policies and stewardship procedures for present and next pandemics has been underlined by a number of recent reviews (9, 21, 22).
2. Objectives
As such, evaluating the use of antibiotics for COVID-19 patients was crucial. The present study aimed to evaluate the efficacy of antibiotic use in COVID-19 patients admitted to a general hospital in Tabriz, Iran, between 2020 and 2021.
3. Methods
Based on data extracted from the medical records office of Sina Hospital, affiliated with Tabriz University of Medical Sciences, this cross-sectional retrospective study analyzed inpatient antibiotic use during the COVID-19 pandemic, focusing on evaluating the rationality of prescribing patterns.
3.1. Antibiotic Utilization
The anatomical therapeutic chemical (ATC) classification system, developed by the WHO Collaborating Center (WHOCC) for drug statistics methodology, was used to categorize systemic antibiotic consumption. Categories included systemic antibiotics (J01), vancomycin (J01XA01), metronidazole (J01XD01), macrolides (J01FA), carbapenems (J01DH), and cephalosporins (J01D) (23). Antibiotic usage was quantified using the defined daily dose (DDD) metric, which represents the average maintenance dose per day for a medicine taken for its primary indication in adults. Antibiotic consumption was expressed as DDD per 1,000 inhabitants per day (DID). The formula used was DDDs/1,000/d = (DDDs used in a year × 1,000)/(population × 365). All historical data were adjusted to the latest WHO DDD/ATC Index to account for changes in recommended doses over time (24).
3.2. Study Design and Population
A total of 1,000 patients were randomly selected for inclusion using computer-generated random numbering in this study, which was carried out at Sina Hospital between March 2020 and March 2021. Approximately 3,000 adult patients with COVID-19 were hospitalized during this period. Patients were eligible if they were 18 years of age or older, had a hospital stay of at least 48 hours, and had a confirmed diagnosis of COVID-19. Of the 1,000 included patients, 947 (94.7%) received antibiotics during their hospitalization. Demographic data, including gender distribution, are presented for these 947 patients.
3.3. Diagnostic Criteria for Coronavirus Disease 2019
Positive PCR test results, chest CT scan results consistent with COVID-19 pneumonia, and/or clinical evaluation were used to confirm the COVID-19 diagnosis. Patients with uncertain diagnoses or insufficient medical records were not included.
3.4. Data Collection
A pre-made checklist including demographic details, comorbidities, and comprehensive antibiotic prescription records was used to retrieve data from patient files. The available records of Medical Records Department of Sina Hospital were used to complete the checklists.
3.5. Diagnosis of Bacterial Infections
Clinical symptoms, lab results, and imaging, where available, were used to diagnose bacterial infections. When it was possible to confirm the diagnosis, positive cultures (such as blood or sputum) were examined. However, empirical antibiotic treatment was frequently given based on clinical suspicion of secondary infections because of early pandemic diagnostic limitations. According to literature findings from comparable cohorts, P. aeruginosa, Mycoplasma pneumoniae, and H. influenzae were the most commonly detected pathogens, when identifiable.
3.6. Statistical Analysis
Following the completion of the checklists and the gathering of all necessary data, the pertinent information was input into IBM SPSS Statistics for Windows (Version 26.0). The data was analyzed using descriptive-analytical tests (Armonk, NY: IBM Corp.). Frequencies and percentages were used to provide descriptive data, whereas the mean and standard deviation (SD) were used to explain quantitative data. To make qualitative comparisons, the χ2 or Fisher exact test were used. A P-value of less than 0.05 on both sides was deemed to indicate statistical significance.
3.7. Ethical Approval
There were no treatment interventions carried out in this retrospective, cross-sectional investigation. Medical ethical norms were followed when reviewing patient files, guaranteeing the privacy of all data. After a thorough evaluation of the thesis’s subject matter and ethical guidelines, the ethics committee of the university gave the thesis its final clearance. The study was approved by the ethical code of IR.TBZMED.REC.1400.1249.
4. Results
The study included 1,000 adult patients with confirmed COVID-19. Among these, 947 patients (94.7%) received antibiotic therapy during hospitalization, and demographic data were analyzed for this subgroup. The average age of the 947 patients was 65 ± 16 years. Of these, 492 (52%) were male and 455 (48%) were female. Most patients (95.35%) had at least one comorbid disease. The most prevalent conditions were diabetes mellitus (256 patients, 27.03%) and hypertension (280 patients, 29.56%) (Table 1).
| Characteristics | No. (%) |
|---|---|
| Age group (y) | |
| 18 to 20 | 48 (5.06) |
| 21 to 40 | 190 (20.06) |
| 41 to 60 | 290 (30.6) |
| 61 to 80 | 349 (36.8) |
| > 80 | 70 (7.39) |
| Sex | |
| Males | 492 (52) |
| Females | 455 (48) |
| Comorbidities | |
| HTN | 280 (29.56) |
| DM | 256 (27.03) |
| HLP | 82 (8.65) |
| COPD | 75 (7.91) |
| DVT | 66 (6.96) |
| CKD | 61 (6.44) |
| Hypothyroidism | 5 (0.5) |
| Psychiatric illness | 17 (1.79) |
| Cancer | 12 (1.26) |
| CHF | 11 (1.16) |
| Anemia | 8 (0.84) |
| Addiction to drugs | 30 (3.16) |
Demographic Characteristics of Hospitalized Patients with Coronavirus Disease 2019 Who Received Antibiotic Therapy (n = 947)
4.1. Number and Type of Antibiotics Used in Patients with Coronavirus Disease 2019
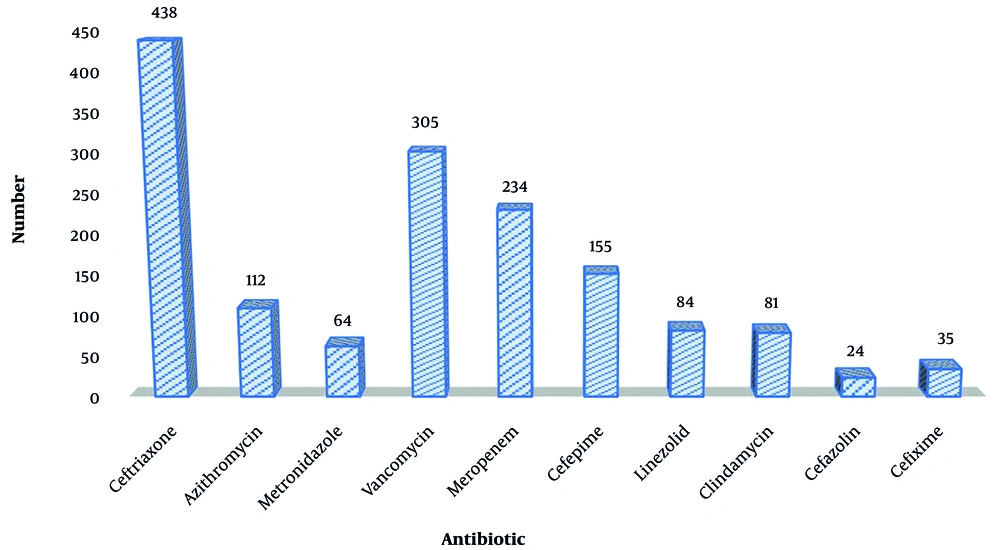
Upon reviewing the medicines used, it was noted that all hospitalized patients were prescribed broad-spectrum medications to prevent hospital-acquired illnesses. The kind of microbe that caused HAI was taken into consideration when choosing medications. Depending on the type and extent of the infection, some individuals had taken two or more antibiotics. Ceftriaxone was the antibiotic that hospitalized patients used most frequently, with 43.8% of patients receiving it. More than 53% of COVID-19 inpatients received a prescription for at least one antibiotic each month (Figure 1).
4.2. Investigating the Rationality of Antibiotic Use in the Treatment of Respiratory Infection of Coronavirus Disease 2019
For qualitative variables, descriptive analyses were performed according to Table 2, and the results were reported as numbers and percentages.
| Variable | Total (n = 1000) | Deceased (n = 283) | Recovered (n = 717) | P-Value |
|---|---|---|---|---|
| Antibiotics | 947 (94.7) | 277 (97.8) | 670 (93.4) | 0.157 |
Number and Percentage of Antibiotic Use in Patients with Coronavirus Disease 2019
Due to clinical uncertainty, the lack of effective antiviral medications at the time, and doctors’ cautious attitude towards treating or preventing any bacterial co-infections, the high rate of antibiotic use (94.7%) during the early pandemic is probably due to these factors. Early Ministry of Health recommendations that allowed the empirical use of antibiotics when secondary illnesses were suspected also had an impact on this prescribing practice. Of the 1,000 patients who were randomly selected for the study, 947 (94.7%) were given antibiotics while they were in the hospital. Table 2 indicates that 277 out of 283 deceased patients (97.8%) also received antibiotic therapy, and 670 out of 717 recovered patients (93.4%) were prescribed antibiotics.
Antibiotic administration was primarily intended to manage suspected hospital- or community-acquired infections and did not appear to have a discernible effect on the progression or resolution of COVID-19 itself, according to statistical analysis that found no significant difference in antibiotic use between recovered and deceased patients (P = 0.157). The most recommended medications for defined daily doses (DDDs) and DDDs per 1,000 DID are shown in Table 3. The DID were 2.044 for patients who passed away and 0.906 for individuals who made a full recovery.
| Antibiotics | ATC Classification | DDD | DID (Recovered) | DID (Deceased) | Consumption (%) |
|---|---|---|---|---|---|
| Ceftriaxone | J01DD04 | 2 | 0.016 | 0.048 | 46.2 |
| Vancomycin | J01XA01 | 2 | 0.026 | 0.053 | 32.2 |
| Meropenem | J01DH02 | 3 | 0.053 | 0.1 | 24.7 |
| Cefepime | J01DE01 | 0.4 | 0.008 | 0.032 | 16.3 |
| Azithromycin | J01FA10 | 0.3 | 0.009 | 0.031 | 11.8 |
| Linezolid | J01XX08 | 1.2 | 0.063 | 0.1 | 8.87 |
| Clindamycin | J01FF01 | 1.8 | 0.072 | 0.37 | 8.55 |
| Metronidazole | J01XD01 | 1.5 | 0.11 | 0.14 | 6.75 |
| Cefixime | J01DD08 | 0.4 | 0.039 | 0.15 | 3.69 |
| Cefazolin | J01DB04 | 3 | 0.51 | 1.02 | 2.53 |
| Total | - | 15.6 | 0.906 | 2.044 | - |
The Inpatient Utilization of Antibacterial Agents for Systemic Use (Anatomical Therapeutic Chemical Group J01), Expressed Inhabitants per Day
According to ATC categorization, the three most often used antibacterial medications for systemic use (J01) during the entire month of observation were meropenem (J01DH02) at 24.7%, vancomycin (J01XA01) at 32.2%, and ceftriaxone (J01DD04) at 46.2% (Table 3).
When it came to the ratio of antibiotic usage compliance with the standard recommendation, azithromycin had the greatest compliance percentage (98 ± 2%) among the used antibiotics, followed by cefazolin and ceftriaxone (97 ± 2%). Table 4 shows that the average percentage of antibiotics used that complied with the standard recommendation was 95 ± 2%. The degree to which the types, dosages, and durations of antibiotics prescribed matched the national COVID-19 treatment guidelines is known as the compliance percentage. The majority of the small percentage of antibiotic prescriptions deemed illogical resulted from dose or duration variations from the advised guidelines. These variations were probably brought about by the lack of culture-confirmed infections, clinical judgment in cases that were unclear, or unclear initial stewardship controls in the early stages of the pandemic.
| Antibiotics | Dose | Duration | The Ratio of the Compliance of the Order with the Guideline (± 2%) |
|---|---|---|---|
| Ceftriaxone | 1 g | 4 - 14 d | 97 |
| Vancomycin | 1 g | 2 - 6 wk | 96 |
| Meropenem | 1 g | 7 - 14 d | 96 |
| Cefepim | 1 g | 7 - 10 d | 94 |
| Azithromycin | 500 mg | 3 - 10 d | 98 |
| Linezolid | 400 - 600 mg | 10 - 28 d | 96 |
| Clindamycin | 600 mg | 7 - 10 d | 94 |
| Metronidazole | 500 mg | 7 - 10 d | 92 |
| Cefixime | 400 mg | 7 - 10 d | 96 |
| Cefazolin | 1 g | 10 - 14 d | 97 |
Choosing the Right Type of Antibiotic, Dose and Duration of Use and Obtaining the Ratio of Compliance the Orders with the Standard Guideline
5. Discussion
This retrospective study’s findings demonstrated that bacterial coinfection was common in COVID-19 patients, which is associated with an increased risk of inpatient death (2). We show that nearly all the COVID-19 patients who were admitted to our hospital had an antibiotic prescription. This is more than we believe is necessary given the weak evidence of bacterial co-infection in this viral disease (25). Clinical confusion, a lack of diagnostic facilities, and early national and international recommendations that encouraged empirical antibiotic use in suspected co-infections all likely contributed to this extensive use (22). The American Thoracic Society and the Infectious Diseases Society of America state that "initial prescribed for adults with clinical and radiographic evidence of CAP who test positive for influenza in the inpatient settings" is the appropriate use of antiviral and conventional antibacterial therapy (26).
A retrospective, multi-center cohort study was carried out to investigate how the usage of antibiotics affected the recovery from COVID-19 infection. Every adult inpatient (over the age of eighteen) from Jinyintan and Wuhan Pulmonary hospitals, Wuhan, China who had COVID-19 laboratory confirmed and been released from treatment or had passed away by January 31, 2020, was included in the study. Among the patients admitted to the hospital, 128 (93%) and 181 (95%) belonged to the category of non-survivors. There were no discernible variations in the usage of antibiotics between survivors and non-survivors (P = 0.15) (27).
COVID-19 patients admitted to Tan Tock Seng Hospital and the National Center for Infectious Diseases in Singapore were the subjects of an observational cohort study conducted from January to April 2020. The patient was deemed to be on empiric antibiotics if COVID-19 therapy was started within three days of diagnosis. Antibiotic medication was not significantly associated with lower 30-day adjusted odds ratio [aOR: 19.528, 95% confidence interval (CI): 1.039 - 367.021] or in-hospital mortality (aOR: 3.870, 95% CI: 0.433 - 34.625) rates after adjusting for age, co-morbidities, and severity of COVID-19 illness (28).
Antibiotics were utilized in 947 (94.7%) of the patients in our study; 277 (94.8%) of them died, and 670 (93.4%) of them recovered. Regarding the improvement of COVID-19 symptoms, there was no discernible difference between the two patient groups (recovered and deceased) (P = 0.157). Ten African nations (Ghana, Kenya, Uganda, Nigeria, South Africa, Zimbabwe, Botswana, Liberia, Ethiopia, and Rwanda) reviewed their national COVID-19 treatment guidelines in order to determine the implications for the continent’s response to AMR. Several medicines, including amoxicillin, ampicillin, gentamicin, benzylpenicillin, piperacillin/tazobactam, ciprofloxacin, ceftazidime, cefepime, vancomycin, meropenem, and cefuroxime, were found to be recommended for use in the management of COVID-19, according to the review (29).
Antibiotics are advised for treating ventilator-associated pneumonia in critically sick COVID-19 patients, preventing secondary bacterial infection, and treating strongly suspected pneumonia based on clinical symptoms in moderate-to-mild COVID-19 patients. Given that COVID-19 is a virus and that only a small proportion of its victims would also have bacterial co-infection, this is worrying (30). Beneficiaries having a COVID-19 outpatient visit and related antibiotic prescriptions were found through Part D event files and 100% Medicare carrier claims, according to a research letter. Those 65 years of age or older who visited between April 2020 and April 2021 and had fee-for-service + Part D coverage were included. Therefore, 346,204 (29.6%) of the 1,169,120 COVID-19 outpatient visits were linked to an antibiotic prescription; azithromycin was the most often prescribed antibiotic (50.7%), followed by doxycycline (14.0%), amoxicillin (9.4%), and levofloxacin (6.7%) (31).
It may be possible to approximate the proportion of the study population that uses a particular drug or class of pharmaceuticals on a daily basis using sales or prescription data reported as DIDs (32). In this study, the chi-square test showed no differences in DIDs between recovered (0.906) and deceased (0.2044) patients (P > 0.05). Ceftriaxone (J01DD04), vancomycin (J01XA01), and meropenem (J01DH02) were the most often used antibiotics in our study, with 46.2%, 32.2%, and 24.7% usage rates, respectively, depending on the kind of acquired secondary infection and the hospital’s antibiotic supply. These results are probably connected to a 2020 study conducted in the intensive care unit (ICU) at the university hospital in Pristina, Kosovo, which discovered that in 17 cases out of 52 COVID-19 patients (32.7%), a significant amount of empirical antibiotics was used to treat atypical pathogens and methicillin-resistant Staphylococcus aureus (MRSA) infections with broad-spectrum antibiotics like ceftriaxone/cefotaxime plus macrolide (7).
Given that COVID-19 is primarily a condition caused by viruses, national guidelines for treating it ought to strongly emphasize the cautious use of antibiotics. Antibiotics must be used carefully, ideally in conjunction with bacterial culture and antimicrobial susceptibility testing, especially broad-spectrum drugs like meropenem (33). The types of antibiotics, their dosages, durations of usage, and levels of conformity with standard guidelines were assessed in order to examine the rationale of antibiotic use. As previously mentioned, the degree to which antibiotic prescriptions (kind, dosage, and duration) adhered to the Ministry of Health’s national COVID-19 treatment guidelines is known as the compliance percentage.
The results showed that azithromycin had the highest compliance percentage (98 ± 2%) with the recommendation, whereas the average antibiotic used with the guideline had a compliance percentage of 95.6 ± 2%. The few examples of irrational usage were largely caused by dosage or duration deviations, which were probably brought on by early uncertainty in guidelines, a lack of microbiological proof, or clinician preference in cases that were severe or unclear (21). Most patients with COVID-19 can be treated without the need for specialized antiviral or antibiotic drugs, according to the diagnostic therapeutic flowchart for COVID-19 (DTFC) in IRAN. In accordance with the patient’s clinical situation, antibiotic therapy should only be initiated when there is a strong suspicion of a bacterial co-infection. The results of the antibiogram and culture, along with the local microbial resistance pattern, may be considered. It is actually not necessary or recommended to use antibiotics when treating COVID-19. Make a judgment based on the recommendation of a specialist physician if a patient needs a prescription for it.
5.1. Conclusions
It is highly advised to avoid prescribing antibiotics unless there is a strong suspicion of concurrent bacterial infections, as they have no demonstrated antiviral effects, exacerbate AMR, and reduce antibiotic efficiency. In nearly all of the COVID-19 inpatients with HAI and CAI in our study, the kind, dosage, and duration of antibiotic administration were appropriate and rationally chosen. The three most frequently given antibiotics were ceftriaxone, vancomycin, and meropenem.
5.2. Limitations
There were certain restrictions on our investigation. Our study did not examine the clinical symptoms, laboratory, or imaging data of COVID-19 outpatients. Patients who were younger than 18 years old or who had been hospitalized for less than 48 hours were not allowed to participate in the trial. Patients who were released from the research with their own consent did not receive the full course of treatment; their data was not incorporated into the study at any point; statistical analyses were also not conducted for them. Furthermore, the results may not apply to healthcare systems with other antibiotic stewardship frameworks or to later stages of COVID-19 management because the study was limited to a single hospital during the early pandemic phase.