1. Background
Staphylococcus aureus are gram-positive aerobic bacteria, and thusthe most important species of genus Staphylococcus. They are dangerous pathogenic bacteria (1). They could produce a golden carotenoid pigment, called staphyloxanthin (it produces yellow colonies). This pigment plays a role in the pathogenesis, because it acts as an antioxidant and protects the bacteria against oxygen free radicals (2). Oxygen free radicals are produced by the host immune system for killing the bacteria (3). Staphylococcus aureus causes a wide range of infections, from simple skin infections to very serious diseases (such as pneumonia, meningitis, and endocarditic). It is known as a common cause of hospital infections, particularly wound infections after surgery (4). Every year, 500 thousand people in hospitals of United States of America are infected with Staphylococcus aureus. It is resistant to Methicillin antibiotic (MRSA) (5). Staphylococcus aureus was first isolated from a pus sample by a Scottish surgeon, Alexander Ogston, in Scotland in 1880 (6). Listeria monocytogenes food are non-sporulation, non-branched, regular, short, anaerobic gram-positive bacteria that are isolated from soil, animal food, water, manure, meat, milk and dairy products, and vegetables (7). These bacteria cause listeriosis disease, which are common diseases between human and animals that cause primary meningitis in adults. Older patients or those with low cellular immunity are particularly susceptible to these bacteria, such as organ transplant recipients or AIDS patients (8). Listeria monocytogenes effect the central nervous system, and causes serious diseases, usually with a high mortality rate. In such a way that in case of food contamination with this bacteria, the mortality rate reaches 40% to 30% and it has been reported that in susceptible individuals, it reaches up to 75%. Among those who have recovered from this disease, the neurological symptoms remain for a long time in the patients (9). Pregnancy increases the risk of listeriosis. Listeria monocytogenes in pregnant females usually causes a bacterial disease like flu, and if it is left untreated, it could lead to inflammation of placenta or amniotic membrane, and fetus’s infection and finally abortion or premature birth (10). The most important way of transfer is through foods. Since these bacteria could be found everywhere, to prevent infections, further control and monitoring of food production and distribution cycle is necessary. The potential risk of contamination with these bacteria has been demonstrated in meat products, raw and pasteurized milk in many studies from different countries (11). The situation of L. monocytogenes is unknown in Iran and little information is available about the presence of L. monocytogenes in food products that are consumed in Iran. Iranians’ eating habits are also different from those in the West, so there is lower risk of infection with this bacteria in Iran (12). Essential oils are volatile compounds that are obtained through various methods, such as distillation, extraction or distillation in a vacuum by solvent extraction plants. Essential oils are aromatic substances that have a strong odor and smell, and chemically contain aromatic ring and benzene and their main ingredients are phenol and oxygen (13). Natural essential oils and their components are the most effective agents of microbial compounds that due to their high purity during preparation, play an important role in controlling microorganisms. In terms of chemical structure, essential oils are a mixture of esters, aldehydes, alcohols, acetone and terpenoids, the components of which are different depending on the plants (14). In addition, these materials are found in different parts of the plant, such as bark, roots, leaves, stems, fruit, seeds, and flowers. Essential oils prevent the production of DNA, RNA, proteins, and polysaccharides in fungal and bacterial cells (15). Secondary metabolites of medicinal plants, such as essential oils and plant extracts, have been studied for their anti-microbial effects. It has become clear that most essential oils that are extracted from herbal plants have anti-fungal, anti-parasitic, antibacterial and anti-virus properties. Thus, identifying plants that have antibacterial properties, and separation and purification of their compounds are effective in the treatment of infectious diseases, and could be a useful way to treat infections resistant to antibiotics. Also, the role of essential oils in a number of medicinal plants to prevent the uncontrolled growth of cancer cells has been demonstrated (16).
Eshvark is a plant with the scientific name of Rhazya stricta, a shrub with a height of 50 to 100 cm from the Apocynaceae species that grows in hot and dry areas. Eshvark is a small shrub with a central soft stem and no fluff. This plant is an indigenous shrub of Sistan and Baluchistan and Hormozgan provinces of Iran (17). It also grows in other countries, such as Afghanistan, Pakistan, Saudi Arabia, and India (18). It’s Fresh and green fruits could be used for treatment of sunburn and sunstroke in the eye. Its corolla is used to quench thirst in the desert. The plant extracts could be used in the treatment of diseases, such as diabetes, fever as well as wounds treatment and to improve liver inflammation (19). Antioxidant properties of this plant has been studied in mice. Studies have shown that its leaves and extracts change enzyme activity in Transaminase Aspartate (AST) and Alanine Transaminase (ALT), and in mice cells, they cause antioxidant activity (20).
2. Objectives
The aim of this study was to initially evaluate the antimicrobial effects of ethanol extract and essential oil of Eshvark against clinical isolates of S. aureus and L. monocytogenes bacteria that are resistant to antibiotics. Finally, inhibitory and deadly concentrations on different strains for the purpose of introduction to the pharmaceutical industry, was determined.
3. Methods
3.1. Plant Material
The leaves of Rhazya stricta were collected from Iranshahr, southeastern Iran, and dried at room temperature. Samples were crashed and transferred to glass containers and preserved until the extraction procedure was performed in the laboratory.
3.2. Distillation of Essential Oils
Plant materials were subjected to steam distillation for 3 hours using a Clevenger-type apparatus. Essential oils were collected after decantation. The distilled essential oil was dried over anhydrous sodium sulfate and was filtered and stored at 4°C.
3.3. Isolation of Bacteria and Culture Method
Different strains of S. aureus bacteria used in this study were isolated from Bu-Ali hospital in Zahedan; they were cultured and purified on special culture medium Mannitol Salt agar and blood agar (MERCK- Germany). These bacteria were then fully identified by using biochemical tests, such as catalase, coagulase, and DNase, then by growth on differential medium. Standard strains of L. monocytogenes (PTCC 1630) were provided as a lyophilized vial at an Iranian collection center of fungus and bacteria (Iranian research organization for Science and industrial).
3.4. Antimicrobial Testing of Essential Oil
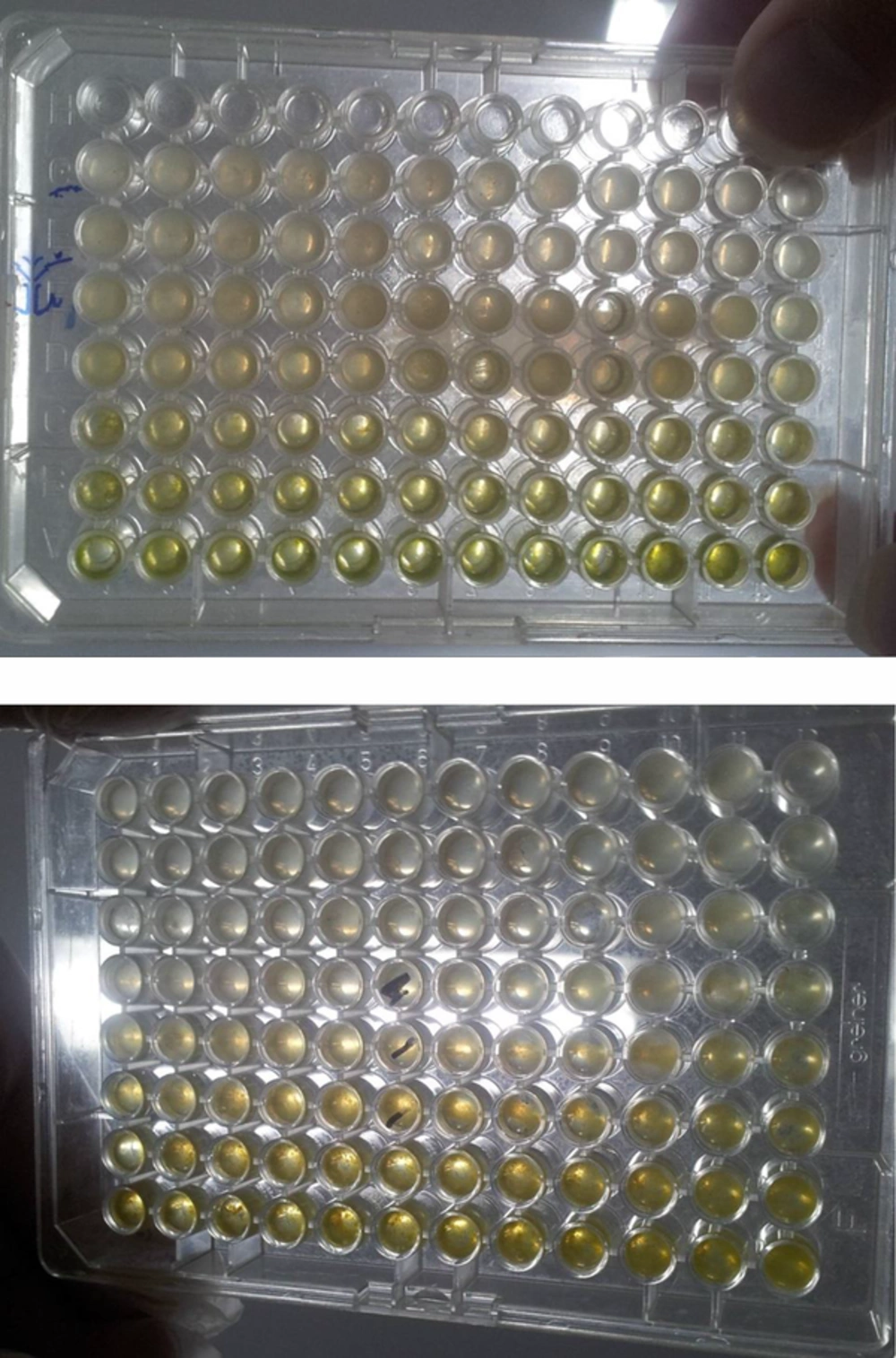
Susceptibility of bacterial isolates with multiple resistances to the plant essential oils of R. stricta was investigated using the dilution plate. One hundred milliliters of nutrient broth Mueller Hinton broth (MHB) was added to seven micro-titter plates. The first well received 100 mL of the diluted solution of the extract, after mixing, and 100 mL of the first plate was removed and added to the second plate, and this was carried on for the entire plate. From the last plate, 100 mL of culture medium containing 107 units per mL was removed, and 100 mL of bacterial suspension equivalent to 0.5 McFarland was added to all wells and incubated at 37°C for 24 hours. The first well that prevented the growth of bacteria was considered as MIC and to ensure this finding, 10 mL of the clear plate was transferred to the Mueller Hinton agar medium. After 24 hours, the first dilution that could kill 99.9% of bacteria showed the Minimum lethal concentration
3.5. Sensitivity Testing of Microbes Against Antibiotics
Sensitivity of 15 strains isolated from 2 species of S. aureus and L. monocytogenes bacteria to ampicillin, tetracycline, erythromycin, penicillin, and cefoxitine antibiotics that were obtained from Padtan Teb-e-Iran pharmaceutical company, were assessed. They were evaluated using the standard Kirby-Bauer disk diffusion method. To do so, all strains of bacteria with concentration of 5.0 McFarland (cfu/mL 108x 1) on Mueller Hinton broth were prepared and then cultured on Mueller Hinton agar. The target disks, according to the manufacturer's instructions, were stained with every antibiotic and then were dried in sterile conditions Finally, they were placed on the surface of Mueller Hinton solid medium containing bacteria. Then, each plate was placed at 37°C for 24 hours, assessing and determining their resistance or sensitivity to antibiotics. Each experiment was repeated 3 times, and the results were analyzed using SPSS version 19 (USA) statistical analysis. The results of this analysis were compared with those of the national committee for clinical laboratory standards (NCCLS).
The inhibitory growth zones that are made by the action of antibiotics on petri dish, are observed as a corona (halo). They could be measured by a ruler. If the size of the inhibition zone around each whole (pit) is more than one centimeter, it is recorded as an acceptable antibiotic activity.
| Inhibition Zone Diameter for Each Strain Against Different Antibiotics, cm | Bacterial Strain | ||||
|---|---|---|---|---|---|
| AMP | TET | ERT | PEN | CEF | S. aureus |
| 1.1 | 1 | 1.5 | 1.2 | 1.7 | 1 |
| 1.9 | 1.2 | 1.7 | 1.7 | 1.1 | 2 |
| 1.9 | 1.1 | 1.1 | 2 | 1.2 | 3 |
| 1.9 | 1.3 | 1.5 | 1.3 | 2 | 4 |
| 2 | 1.7 | 1.9 | 2 | 1.9 | 5 |
| 1.8 | 1 | 2 | 2 | 1 | 6 |
| 1.8 | 1.2 | 1 | 2 | 2 | 7 |
| 1.7 | 1.7 | 1.1 | 1.9 | 2 | 8 |
| 1.8 | 1.7 | 1 | 1.9 | 1 | 9 |
| 2 | 1.7 | 1.5 | 1.1 | 1.5 | 10 |
| Inhibition Zone Diameter for Each Strain Against Different Antibiotics, cm | Bacterial Strain | ||||
|---|---|---|---|---|---|
| AMP | TET | ERT | PEN | CEF | L. monocytogenes |
| 1.2 | 2 | 1.3 | 1 | 1.5 | 1 |
| 1.5 | 2 | 1 | 1.2 | 1 | 2 |
| 1.6 | 2 | 1.2 | 1.1 | 1.9 | 3 |
| 1 | 2 | 1.7 | 1.7 | 1.5 | 4 |
| 1.7 | 2 | 1.2 | 1 | 1.6 | 5 |
| 1.6 | 1.3 | 1 | 1.8 | 1.7 | 6 |
| 1.1 | 1.2 | 1.1 | 2 | 1.7 | 7 |
| 1.7 | 1.4 | 1 | 1.9 | 1.1 | 8 |
| 1.7 | 1.2 | 1.7 | 1.3 | 1 | 9 |
| 1 | 1.2 | 1.3 | R | 1.5 | 10 |
3.6. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
The broth micro dilution method was used to determine MIC and MBC (25). All tests were performed by Mueller Hinton broth, supplemented with Tween 80 at a final concentration of 0.5% (v/v). Briefly, serial doubling dilutions of the extract were prepared in a 96-well micro titer plate ranging from 0.3 mg/mL to 10.00 mg/mL. To each well, 10 μL of indicator solution (prepared by dissolving a 10-mg extract in 2 mL of DMSO) and 10 μL of Mueller Hinton Broth were added. Finally, 10 μL of bacterial suspension (106 CFU/mL) was added to each well to achieve a concentration of 104 CFU/mL. The plates were wrapped loosely with cling film to ensure that the bacteria did not become dehydrated. The plates were prepared in triplicates, and were then placed in an incubator at 37°C for 18 to 24 hours. The color change was then assessed visually. The lowest concentration at which the color change occurred was taken as the MIC value. The average of 3 values was calculated providing the MIC and MBC values for the tested extract. The MIC was defined as the lowest concentration of the extract at which the microorganism does not demonstrate visible growth. The microorganism growth was indicated by turbidity. The MBC was defined as the lowest concentration of the extract at which the incubated microorganism was completely killed.
4. Results
4.1. The Reaction of Two Species of Bacteria to the Antibiotics
Antibacterial activities of several antibiotics were evaluated against 2 strains of S. aureus and L. monocytogenes under laboratory conditions. The results showed that these 2 bacterial strains almost showed identical behavior, yet demonstrated some differences as well. For example, 8 strains of S. aureus bacteria showed resistance to 3 antibiotics, and the fifth strain showed the highest resistance to all antibiotics (Table 3). The sensitivity or resistance of strains of L. monocytogenes showed that 4 strains were resistant to 3 antibiotics, but most strains were resistant to 2 antibiotics. Strain number 4, demonstrated the highest level of resistance to 4 antibiotics (Table 4). Among the antibiotics, ampicillin had the lowest inhibitory concentration for the growth of S. Aureus, which is indicative that 95% of strains were resistant to this antibiotic, and strain number 1 demonstrated intermediate level of resistance (Table 3). The two antibiotics, ampicillin and cefoxitin showed the lowest inhibitory effect for the growth of L. monocytogenes (Table 4).
| S. aureus | Antibiotics | ||||
|---|---|---|---|---|---|
| AMP | TET | ERT | PEN | CEF | |
| 1 | I | S | R | I | R |
| 2 | R | I | R | R | S |
| 3 | R | I | I | R | I |
| 4 | R | I | R | I | R |
| 5 | R | R | R | R | R |
| 6 | R | S | R | R | S |
| 7 | R | I | S | R | R |
| 8 | R | R | S | R | R |
| 9 | R | R | S | R | S |
| 10 | R | R | R | S | R |
| L. monocytogenes | Antibiotics | ||||
|---|---|---|---|---|---|
| AMP | TET | ERT | PEN | CEF | |
| 1 | I | R | I | S | R |
| 2 | R | R | S | I | S |
| 3 | R | R | I | S | R |
| 4 | S | R | R | R | R |
| 5 | R | R | I | S | R |
| 6 | R | I | S | R | R |
| 7 | S | S | I | R | R |
| 8 | R | I | S | R | S |
| 9 | R | S | R | I | S |
| 10 | S | S | I | R | R |
4.2. The Evaluation Results of the MIC and MBC
The results of MIC and MBC extracts on two bacterial strains used in this study are shown in Tables 5 and 6.
| Extracts Concentration | 0.5 | 1 | 1.5 | 2 | 2.5 | 5 | 10 |
|---|---|---|---|---|---|---|---|
| MIC | 0 | 0 | 0 | 5.5 | 7.1 | 83.25 | 17.6 |
| MBC | 0 | 0 | 0 | 0 | 5.5 | 7.1 | 94.11 |
| Extracts Concentration | 0.5 | 1 | 1.5 | 2 | 2.5 | 5 | 10 |
|---|---|---|---|---|---|---|---|
| MIC | 0 | 0 | 0 | 5.3 | 8.5 | 53.5 | 7.1 |
| MBC | 0 | 0 | 0 | 0 | 5.3 | 8.5 | 77.7 |
The results of these tests showed that the highest sensitivity concentrations were 5 and 10 mg/L in S. aureus, 2.5 and 5 mg/L in L. monocytogenes (Tables 3 and 4). The turbidity of the culture medium containing bacteria that was affected by Eshvark extract was also significantly reduced. The rate of change in turbidity was caused by decrease in the growth of bacteria in culture. The results of these tests show that the Eshvark extract has greater inhibitory effect on S. aureus than L. monocytogenes.
5. Discussion
Today, with wide spread bacterial resistance, the world is confronted with various antibiotics (21). Therefore, obtaining more knowledge about plant extracts and compounds and their application is important to deal with pathogenic bacteria. This study also showed that S. aureus is resistant to the antibiotic ampicillin. It was also found that L. monocytogenes is resistant to cefoxitin antibiotics. One way of solving this problem is the study of substances that are found in plants (22). In the recent study the antibacterial effect of Eshvark extract on some bacteria like E. coli has been investigated, and the results were indicative of positive effect of this plant on these bacteria (17). Due to the favorable impacts of this plant, in the current study the effect of ethanol extract of the plant on two bacteria of S. aureus and L. monocytogenes and its role in the treatment of diseases caused by these bacteria were studied. Regarding the reaction of bacterial strains to antibiotics, tests showed that all strains reacted similarly to a special antibiotic; this is indicative of low genetic diversity of strains in populations under study. However, the strains responded differently to various types of antibiotics and this is due to the type and mode of action of antibiotics on specific locations within the cell, such as mitochondria, plasma membrane, and the nucleus. The results of this study showed that the inhibitory effect of Eshvark extract on S. aureus was greater than that of L. monocytogenes. This could be due to a stronger cell wall in L. monocytogenes (23). The existence of free radicals in the extract of this plant could also be effective in inhibiting bacteria and the ethyl acetate compound in the extract could create maximum inhibitory effect (24). In a study conducted in China, the effect of 19 drug species, against hospital strains of S. aureus showed that the concentration of this herbal extract with a minimal inhibitory effect was at 10 mg per mL (25). These tests correspond with the findings of the current research. In another study, the antimicrobial effect of essential oils of several plant species on L. monocytogenes was investigated. The results showed that the essential oil of cloves and Hindi mint had good effects against the bacteria (26, 27). However, studies showed that the effect of the essential oils on E. coli was greater, because E. coli bacteria is gram negative and L. monocytogenes is gram positive. Therefore, it demonstrated greater sensitivity to these essences. This is probably due to the differences in the strength of the cell wall of gram-negative and gram-positive bacteria and lack of membrane in gram-positive bacteria (23). It is important to know that the compositions in plant extracts may introduce the most effective agent in the extract. Taking into consideration the diversity of these compositions, analysis of these compounds by means of various chromatographies could identify effective compounds and introduce them to the pharmaceutical industry.
After general introduction of a plant to deal with the disease caused by bacteria, further studies are required to determine the active ingredients of that plant. It should also be noted that due to strains resistant to common antibiotics, it is necessary to use a lower dose for each patient, and so, the extract of boiled Eshvark must be administered to the patient for favorable impact on controlling the disease.