1. Background
Increase in the immunocompromised population is highly important and results in an increase of opportunistic infections. In this context, invasive fungal infections (IFI) have gained great importance due to their high incidence and high morbidity and mortality rate. Most of them are produced by Candida spp although the incidence of infections with filamentous fungi such as Aspergillus spp is also high. The clinical risk factors of IFI can lead to confusion because it is a disease without obvious specific symptomatology, and thus many of its symptoms coincide with those of any type of infection or serious illness in critically ill patients (1).
The main obstacle to determine the exact extent of the infection lies in its diagnosis. The difficulty is due to the diversity of the microorganisms that can cause the disease as well as the limits in the currently available techniques that often postpone the most appropriate antifungal treatment and favour the irreversible organ damage (2-6).
Nuclear medicine is the branch of medicine that uses radioactive compounds for diagnostic or therapeutic purposes. One of the major comparative advantages of diagnostic nuclear medicine, unlike other imaging techniques, is that it provides functional information on pathophysiological and biochemical processes, differentiating an abnormal anatomy, physiology, or biochemistry from normal anatomy based on the distribution pattern in the body of radioactive tracers, commonly called radiopharmaceuticals. Despite the many efforts dedicated to the development of a radiopharmaceutical with adequate sensitivity and specificity to distinguish between infection and sterile inflammation, the ideal agent has not yet been achieved. Nuclear medicine non-invasive imaging techniques anatomically outline the main sites of infections, and as a result, invasive biopsy procedure can be avoided. Radiopharmaceuticals may be used as diagnostic agents for these pathologies, helping to identify and localize hidden infection foci. Thereby, obtaining a specific tracer that allows the identification of mycotic foci may be useful to start the treatment before the dissemination of the IFI in immunosuppressed patients. An ideal radiopharmaceutical for the detection of infection must meet the following criteria:
- High sensitivity and specificity for infection
- Low or no toxicity
- Rapid absorption in inflammatory lesions
- Low physiological uptake in blood and organs
- Rapid elimination
- Availability, ease of use, and low cost
Despite the remarkable growth of the positron emission tomography, 99mTc continues to occupy a prominent place in diagnostic nuclear medicine mainly due to its wide availability and low cost. As a transition metal, it has a rich coordination chemistry, which offers many possibilities to label biologically active molecules (7).
Antifungal molecules are a valid option, as they are directed to a specific target of action like being the microorganisms that cause the pathology to be diagnosed. Amphotericin B (AMB) may be a good candidate for this purpose because its polyene structure allows forming complexes with sterols of the cell membrane of the fungus.
Sternal et al. suggested that 7 or 8 molecules of AMB and sterol form a transmembrane pore in which the polar aminoglycoside part is oriented towards the surface with the hydroxyl groups towards the centre of the channel, and the polyene chain interacts through van der Waals forces with the ergosterol molecule and membrane phospholipids. Hence, it would be possible to form coordination complexes with 99mTc from the aminoglycoside group without affecting the binding to sterols (8-11).
The fac (99mTc)((CO)3(H2O)3)+ complex, developed by Agorastos et al. (12), can be used as a precursor for the preparation of a variety of potential radiopharmaceuticals. This complex has 3 coordination positions occupied by molecules of CO strongly bonded to the metal. The other available coordination positions are occupied by 3 weakly bound water molecules, which may be replaced by donor atoms with high affinity for the metal.
Within the Tc compounds, the derivatives of the Tc(V)-Nitride are outstanding. These compounds are characterized by the presence of a triple terminal bond, Technetium ≡ Nitrogen that leaves 4 coordination positions available for binding to the ligand (13-19). AMB has amino and hydroxyl moieties, which can act as donor groups to bind to metal complexes as the ones mentioned above.
From the practical point of view, the preparation of the complexes containing 99mTc precursors is performed by a reaction in 2 stages: reduction of pertechnetate, followed by substitution by the appropriate ligands.
2. Objectives
This study aimed at presenting the synthesis of coordination compounds between 99mTc and amphotericin B and their physicochemical and biological evaluation as potential tracers of fungal infections by gamma scintigraphy.
3. Methods
3.1. Reagents
(99mTc) NaTcO4 eluate was from a commercial generator (Tecnonuclear, Argentina). Amphotericin B commercial product (CRISTALIA, Lot 11096548) was used for labelling. The purity controls of the product and the derivatives were performed against a USP Reference Standard for amphotericin B (CAT No. 1032007, Lot: KOH185).
All reagents and solvents used were of suitable analytical grade and commercially available. Aqueous HPLC mobile phases were prepared with freshly distilled water, and were filtered prior to use to remove particles that could be suspended. In all cases, the used mobile phases were degassed by ultrasound immediately before use.
3.2. Synthesis of Tricarbonyl Precursor
In a vial a mixture of 4.0 mg Na2CO3, 20.0mg Na/KC4H4O6, and 7.0 mg NaBH4 was purged with carbon monoxide for 20 minutes. Afterwards, 1.0 mL of Na99mTcO4 was added and heated in a water bath at 60°C to 70°C for 30 minutes. Radiochemical purity (RCP) was assessed and monitored by HPLC 1 coupled to gamma detector (12, 13, 18).
HPLC equipment:
- Equipment: Shimadzu LC-10AS
- U / V Detector: Shimadzu SPD-M10A
- Gamma detector: Parken
- Flow: 1 mL/min; Room temperature
HPLC 1 system:
- Column: Phenomenex C-18 Luna 5 μm of 15 cm.
- Mobile phase A: Phosphate/Triethylamine buffer pH = 2.5. Mobile phase B: methanol.
- Gradient: 0 - 3 minutes, 100% A; 3 - 6 minutes, 100% to 75% A; 6 - 9 minutes, 75% to 66% A; 9 - 20 minutes, 66% at 0% A; 20 - 27 minutes, 0% A; 27 - 30 minutes, 0% to 100% A.
3.3. Labelling of the Ligand with Tricarbonyl Precursor - Complex I
A fraction of 3.0 mg of AMB was incubated with 0.3 mL of tricarbonyl precursor in 0.5 mL of water for injection for 30 minutes at 40°C. The solution was then neutralized with Na2HPO4 390 mg/mL. After purification of the HPLC peak, RCP was assessed using HPLC 1 / gamma (13).
3.4. Synthesis of the Nitride Precursor
A fraction of 5.0 mg of succinic dihydrazine was dissolved with 200 μL of 0.9% NaCl and 150 μL of SnCl2 solution (1.0 mg/mL), then, 1.0 mL Na99mTcO4 was added and incubated at room temperature for at least 20 minutes. Radiochemical purity was assessed by paper radiochromatography (Whatman 1 / Acetone, Rf Precursor = 0.0 - 0.1, Rf 99mTcO4- = 0.9 - 1.0) and HPLC 2 / Gamma.
HPLC 2 system:
- Waters C-18 10 μm 3.9 × 300 mm colum.
- Mobile phase A: 0.1% TFA in H2O. Mobile Phase B: 0.1% TFA in Acetonitrile
- Gradient: 0 to 10 minutes, 100% A; 10 to 20 minutes, 100 to 0% A; 20 to 24 minutes
3.5. Amphotericin Derivatization
To label AMB with nitride precursor, the molecule had to be modified or derivatized to act as a proper ligand to allow the formation of a coordination compound with 99mTc. The derivatization of AMB was done with carbon disulphide under a nitrogen atmosphere and with continuous stirring, 1.0 × 10-4 mol of amphotericin B reacting in basic aqueous medium with 4.0 × 10-4 mol of CS2. The reaction was monitored by HPLC-U / V during 48 hours (19).
3.6. Labelling of Ligand with Nitride Precursor - Complex II
A fraction of 500 μL of the derivatized AMB was added to 200 μL of Nnitride precursor and heated in a water bath at 60°C to 70°C for 60 minutes. RCP was assessed using HPLC 2 system (19).
3.7. Physicochemical Studies
3.7.1. Stability in the Reaction Milieu (SM)
Stability in time of complexes I and II in labelling milieu was evaluated up to 20 hours, post labelling by HPLC using the reference systems mentioned above.
3.7.2. Lipophilicity (Lip)
Lipophilicity was studied through the apparent partition coefficient (P) between n-octanol and sodium phosphate buffer (0.1 M, pH = 7.4). Partition coefficient (P) was calculated as the mean activity value of the organic divided in the aqueous phase; lipophilicity was expressed as log P (n = 2).
3.7.3. Plasma Protein Binding (PPB)
The complex was incubated in a pool of human plasma at 37°C, 50 μL samples were seeded in GE G-50 micro spin columns at 30, 60, and 90 minutes post labeling for size exclusion separation. The PPB% was calculated as the ratio between the net activity eluted divided by the total activity (eluted + retained in the column) (n = 2).
3.7.4. Stability in human plasma (HPS)
In vitro stability of 9 complex I and II was evaluated incubating the samples in the same conditions as mentioned above. Samples of 200 μL were collected at 0.5, 1, 2, and 4 hours post incubation.
Proteins were precipitated with cold absolute ethanol shaken in a vortex mixer and cooled in a freezer for 5 minutes to induce precipitation. After centrifugation at 15,000 rpm for 5 minutes at 4°C, the pellet was carefully separated from the supernatant, and an aliquot was analyzed by HPLC with the reference systems.
3.7.5. Yeast Binding (YB)
Seven serial 2-fold dilutions of a No. 5 Mc Farlan suspension of Candida albicans were prepared. Each dilution was added with 100 μL of the labelled molecule and incubated for 1 hour at 37°C. Then, the total activity of the series was measured and centrifuged at 15,000 rpm for 5 minutes at 4°C to obtain a yeast pellet. The yeast binding percentage was calculated as
(“Net Act. Pellet” / “Total Net Act”) × 100 (n = 2).
3.7.6. Biological Studies (BS)
According to in vitro studies, only complex I presented adequate characteristics to proceed to biodistributions. All procedures were performed according to protocol number 101900-000629-14, approved by the honorary commission of animal experimentation (CHEA) (University of the Republic, Uruguay). Animals were provided by the hygiene institute animal housing (University of the Republic, Uruguay). Our department has an area specially conditioned to work with experimental animals under treatment with radioactive material, according to the sanitary and safety requirements of our institution.
Animals were controlled in detail based on the daily observation of signs including general appearance, hair condition, adopted postures, feeding habits and water intake, and behaviour towards other animals of the box.
Biological studies were performed during February and September 2016. The animal model consisted of CD1 mice, females 8 to 10 weeks of age, and a weight between 22 ± 2 grams (n = 3/group). Biological evaluation was conducted in 4 groups of animals G0 = sham, G1 = sterile inflammation, G2 = Candida albicans infection, and G3 = Aspergillus niger infection. Injury induction involved subcutaneous injection in the left hind thigh according to the conditions described below:
- Group 1: Sterile inflammation induced with 50 µL Turpentine oil (20% v/v), 24 hours before biodistribution studies
- Group 2: Fungal infection induced with 50 µL Candida albicans 1 × 107 cfu fresh culture, 48 hours before biodistribution studies
- Group 3: Fungal infection induced with 50 µL Aspergilus niger 1 × 107 cfu fresh culture 7 days before biodistribution studies
Mice were maintained in hygienic housing conditions, with cycles of 12/12 light / dark hours and temperature of 22 ± 2°C, with food and water ad libitum. Complex I, diluted in saline solution, was injected into lateral tail vein (0.1 mL with a maximum activity of 1.11 MBq (30 μCi), and its biodistribution was studied at 3 and 6 hours post injection. Animals were sacrificed by cervical dislocation and the organs of interest were removed, and urine, blood, muscle, and bone samples were also colected. The percentages of injected doses per organ in sham group were determined to establish the general behaviour: blood clearance, elimination pathways, activity in the elimination organs (kidney, bladder, liver, and intestines) and excreta activity (urine and faeces).
4. Results
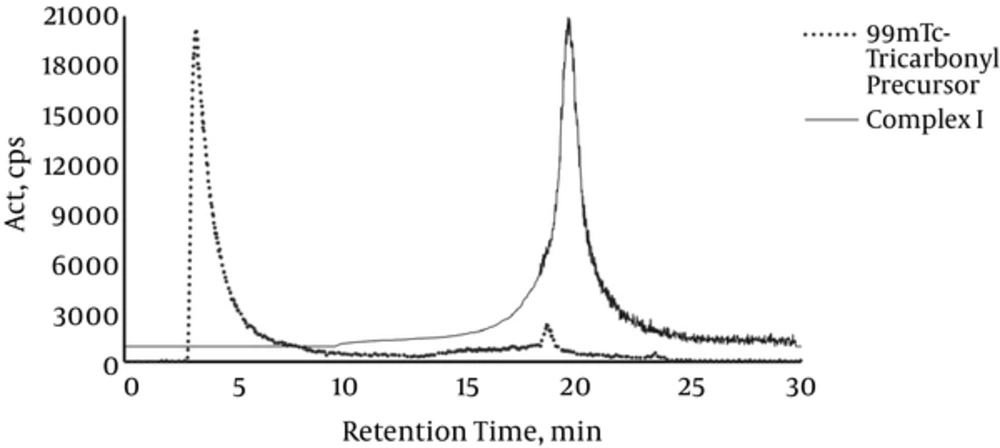
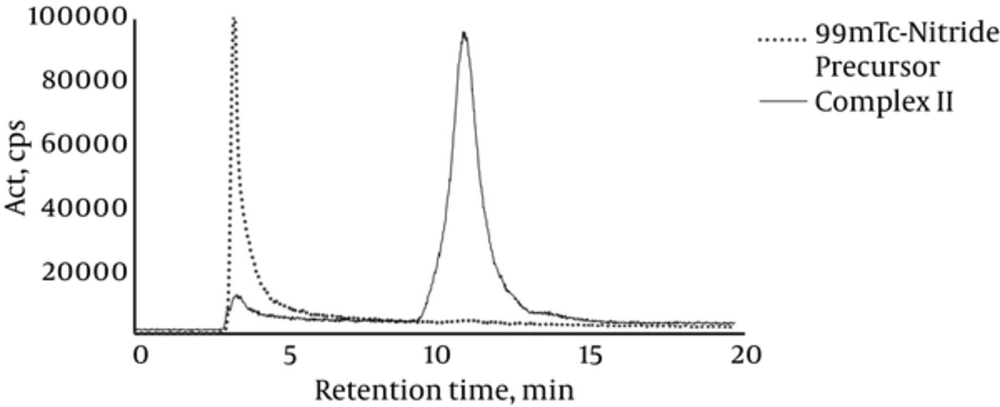
Chromatograms of precursors and complexes are demonstrated in Figures 1 and 2.
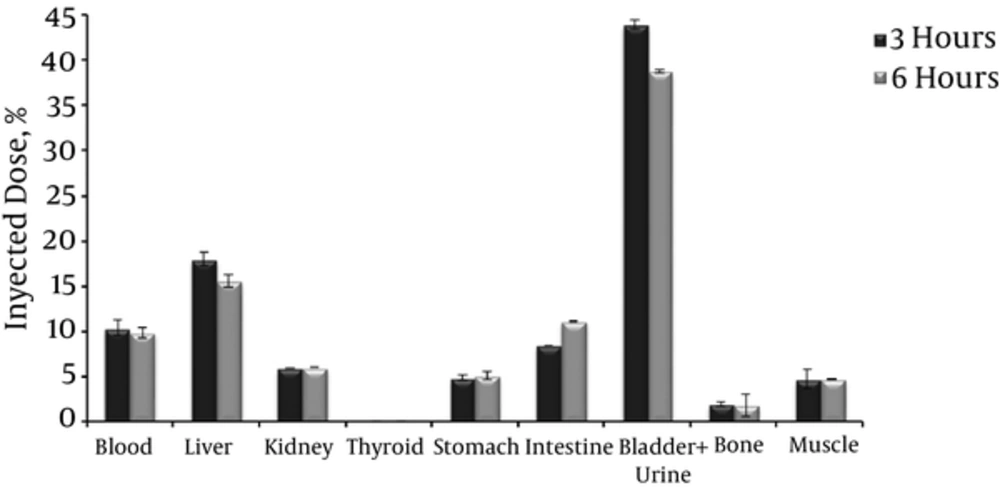
Figure 3 displays the biodistribution profile of complex I in healthy mice at 3 and 6 hours post injection.
Both healthy (nontarget, NT) and injured (target, T) tissues uptake was analyzed according to the following formula:
Target/Non Target = (Act/gram T)/Act/gram NT).
Results are presented in Table 2.
5. Discussion
Both tricarbonyl and nitride precursors were obtained with RCP higher than 90%, as shown in Figures 1 and 2. In the case of complex I, the peak was isolated using HPLC 1 system. A single peak was obtained and its RCP remained greater than 90% up to 20 hours post labeling (Figure 1). On the other hand, Complex II labeling conditions were optimized without requiring further purification steps, obtaining a single peak with RCP higher than 90%, which was stable up to 20 hours post labeling (Figure 2).
Lipophilicity is an important molecular property and is useful for correlating the in vivo behavior of a compound such as membrane permeability, solubility, volume of distribution, metabolic stability, and binding to plasma proteins among others. The values of 0.7 and -1.65 for complex I and II respectivelyshown in Table 1 indicate a greater affinity for the aqueous phase, which would be beneficial, as the compounds could present a predominantly renal elimination and faster purification with the consequent reduction of the patient’s exposure rate to ionizing radiation.
| Variables | Complex I | Complex II |
|---|---|---|
| SM, hs | > 20 | > 20 |
| Lip | 0.71 ± 0.01 | -1.65 ± 0.08 |
| PPB, % | 77.6 ± 0.5 | 53.0 ± 2.8 |
| HPS, % | > 90 | 84.5 ± 5.4 |
| YB, % | 39.9 ± 1.8 | 14.2 ± 1.9 |
The evaluation of plasma protein binding is important because it has a great impact on the pharmacokinetic and pharmacodynamic behavior of a compound, moreover, only the free fraction can access the target sites, and thus identify the pathological areas.
The PPB was 77.6 for complex I and 53.0 for complex II; these are expected values considering that amphotericin B alone has a PPB greater than 90%.
In the tested yeast concentration range, both complexes showed a non-cfu-dependent yeast binding profile, with complex I being clearly the one with higher performance (39.9%) (Table 1). This fact conditioned the decision to choose complex I as more suitable to perform biological studies.
In all 99mTc radiotracers biodistributions, thyroid and stomach act as indicative organs of the stability of the complex in the bloodstream. This is because in vivo reoxidation of the complex to free 99mTcO4- is preferentially accumulated in these organs, so if they present high activity, it could be due to this reason. In this case, the percentage of injected dose in both thyroid and stomach was lower than 5%, which confirms the in vivo stability of complex I (Figure 3).
Biodistribution studies show that the elimination route of the complex is mainly by the urinary tract. Approximately 40% of the injected dose is in bladder and urine, and kidneys presented the highest percentage of injected activity per gram. Data at both 3 and 6 hours show a slow hepatic clearance accompanied (15% - 20%) by a slight increase in intestinal activity (10% - 12%), and the obtained values were consistent with the literature (9, 10), which indicated a liver clearance of 20% to 30% for AMB.
Blood uptake was approximately 10%, muscle and bone presented < 5% of injected dose. A nonstatistical significant difference was observed in the values at the different biodistribution times.
As the aim of this study was to prepare a suitable radiotracer to be used for scintigraphic images, the accumulation of the radiotracer at healthy tissue (nontarget) should clearly differentiate the injury site (target), being infection or sterile inflammation.
Complex I showed a markedly differential uptake for the 3 pathological studied processes: Sterile inflammation, Candida albicans infection, and Aspergillus niger infection (Table 2).
| Target/Non Target Ratio | Value |
|---|---|
| Inflammation G1 | 1.50 ± 0.02 |
| Candida infection G2 | 4.7 ± 0.6 |
| Aspergillus infection G3 | 2.4 ± 0.1 |
As an irritating agent produced sterile inflammation, the aim of this study was to evaluate the nonspecific accumulation of the complex at the target site due only to a greater lymphocytes supply to the injured area for G1, caused by increased irrigation. The highest target/target ratio was observed for infection caused by Candida albicans G2, being 3.1 and 2.0 times greater than sterile inflammation and Aspergillus niger infection, respectively. Complex I presented enough specificity to differentiate between sterile inflammation and infected tissue.
In the current investigation, the feasibility of amphotericin B radiolabeling, using 99mTc- tricarbonyl and 99mTc-nitride precursors and biological evaluation in animal models was assessed to verify the achievability of 99mTc-AMB as a potential in vivo diagnostic agent of fungal infections. Tricarbonylic precursor was obtained to substitute water molecules with AMB; the product, complex I, was characterized both in vitro and in vivo. To synthetize complex II, AMB had to be previously derivatized to form a coordination compound with 99mTc-nitride core. Both complexes showed high radiochemical purity and remarkable in milieu stability. Complex I showed high urinary elimination and moderate and high specificity with good T/NT ratios in G2 and G3 models, respectively, allowing a clear differentiation of injured tissue vs. healthy or inflamed one. Complex I is outlined as a potential agent for rapid and specific diagnosis of infections, caused by candida albicans. Although 99mTc-Tricarbonyl-AMB should be studied in further preclinical stages, this complex, developed by our group, appears as an interesting radiopharmaceutical agent for fungal infection diagnosis.