1. Background
Although fungal infections are less popular than bacterial and viral putridity have been in the past, in recent decades they have been responsible for a dramatic increase in the incidence of disease. In a survey conducted between 1979 to 2000, in America, the incidence of fungal infection has increased 207% (1). In a survey conducted in America between 1997 and 1980 showed that the increased mortality is due to fungal infection and is one of the most common causes of death in the 7th upgraded (2). Predisposing factors, such as broad and long-term use of antibiotics, corticosteroids, immunosuppressive drugs and diseases field, such as diabetes, malignancies, and caused candidiasis than in the past, especially fungal diseases, are on the rise. In a study of Candida species, the 4th factor of morbidity and mortality due to circulatory infections emerged and 35% of bloodstream infections resulting in death have been included (3). The causal agent of opportunistic fungal genus Candida, which cannot coexist in the gastrointestinal tract, mucous membranes, skin, other animals, and even in humans are consistent. The host resistance factors for local or systemic conditions that are primary or secondary in effect reduces patient risk factors, which will be capable of causing disease in any area of the body. There are limitations such as the small number of antifungal drugs, their toxicity to the cells of the body, or decrease sensitivity to these drugs, a series of Candida species, has always been considered as the main complexities in the treatment of disease (4).
T. polium L. (Lamiaceae) has numerous pharmacological properties. These include calcium antagonistic, anorexic, intestinal motility and hypertension, anti - ulcer, anti - inflammatory, antipyretic and antibacterial actions, glycemic and hypo - lipidemic effects, treat liver disease, and diabetes. The extract is also used as an antiemetic, an antispasmodic, an anti-inflammatory, an antipyretic, an analgesic, and an anti-carminative (5). The antibacterial activity of T. polium extracts can be attributed to its contents in flavonoids. In general, the methanol extraction of T. polium plant material yielded to flavonoids (6).
Citrullus colocynthis from the Cucurbitaceae family is used to treat fever, liver, abdominal diseases, and heart problems (6). Root extract is used to treat jaundice - urogenital diseases and rheumatism (7). The seeds are diuretics (8). Its fruit is used in gastrointestinal tumors, causing cancer impairment. In addition, it is effective in leukemia and joint pain. Fluconazole is the most commonly used drug for the treatment of udder candidiasis. This medicine has been studied for the treatment of HIV (9).
Expansion of resistor to fluconazole in the ill has become an adult relevance and is generally dependent with the point of immunosuppression and the collected value of drug (10). Furthermore, Fluconazole has been applied efficiently to treat this infection in the ill and embrace head and neck glow, as the dominant organism has been C. albicans (11).
2. Objectives
The aim of this study was the evolution of susceptibility of strains of Candida albicans isolated from patients to Fluconazole and determination of antibiotic resistance gene.
3. Methods
3.1. Sample Preparation
The plants of T.polium and C.colocynthis were collected from Sistan and Balouchestan province of Zabol city. The leaves of T.polium and C.colocynthis fruits were dried and grinded. A total of 10 g of dried powder of the plant were placed in 100 ml of ethanol, methanol, and ethyl acetate solvents for 24 hours. Finally, they were filtered using filtering paper, and concentrated using a rotary machine, dissolved in a DMSO solvent, and stored in a refrigerator at 4°C.
3.2. Isolation of Fungal
Samples were isolated from women suspected of Candidiasis vaginalis (Amir - al - Momenin Hospital, Zabol, Iran) during 2015 - 16. After receiving the specimen from the vagina with sterilized swabs, the specimens in the Falcon containing 1 cc of the physiology serum were transferred to the laboratory. In the laboratory, vaginal swab specimens were placed in a Sabouraud Dextrose Agar medium and then placed in a 37 - degree incubator for 24 hours.
In this part, in order to prevent the growth of saprophyte fungi, cicloheximide, and to prevent the growth of bacteria, chloramphenicol was added to the Sabouraud Dextrose Agar medium. The samples were taken from a white yeast colony obtained from the Sabouraud Dextrose Agar medium, stained with lactophenele cathene blue, and drained on a Lame. After confirming the colony under a microscope, it was cultured in a chromogen agar culture medium and through the formation of color, Candida albicans species were identified.
3.3. The MIC (Minimum Inhibitory Concentration) and MFC (Minimum Fungicidal Concentration) of the Fungus Were Tested by Using Dilution Method
In order to investigate the antifungal effects of plant extracts, the minimum inhibitory concentration of growth (MIC) and minimum fungicidal concentration (MFC) by microdilution method in 96 parts microplates were done as follows: first, 100 μL sterile from sabouraud dextrose broth medium (Merck, Germany) was poured into a row of microplate wells. Then, 100 μL of the highest concentration of solution of the extract was added to the first well and mixed well. From the 1st well, 100 μL of the solution was taken and added to the next wells containing only 100 μL of the culture medium. This process proceeded from the 2nd to the 3rd well, and so on to the 10th well, to produce all the desired concentrations. In addition, 100 μL of the 10th well was discarded to make the volume of all wells identical. The 11th well was placed as a positive control containing 100 μL of sterile SDB medium and 100 μL sterile liquid medium was poured into a 12th well as a negative control. Further, 10 μL of fungal suspension was added to all wells. Then incubation was performed. A well without turbidity means that the fungus has not grown. The last dilution in which no turbidity was detected was taken as a 10 μL MIC and transferred to a Petri dish containing SDA medium. The concentration in which fungus did not grow on the culture medium was considered as MFC (12).
3.4. Statistical Analysis
Statistical analysis was performed using SPSS version 15, the mean differences analysis was done, and tables were drawn with Excel 2010 software. Results were evaluated at a significant level of P < 0.001.
3.5. PCR Amplification
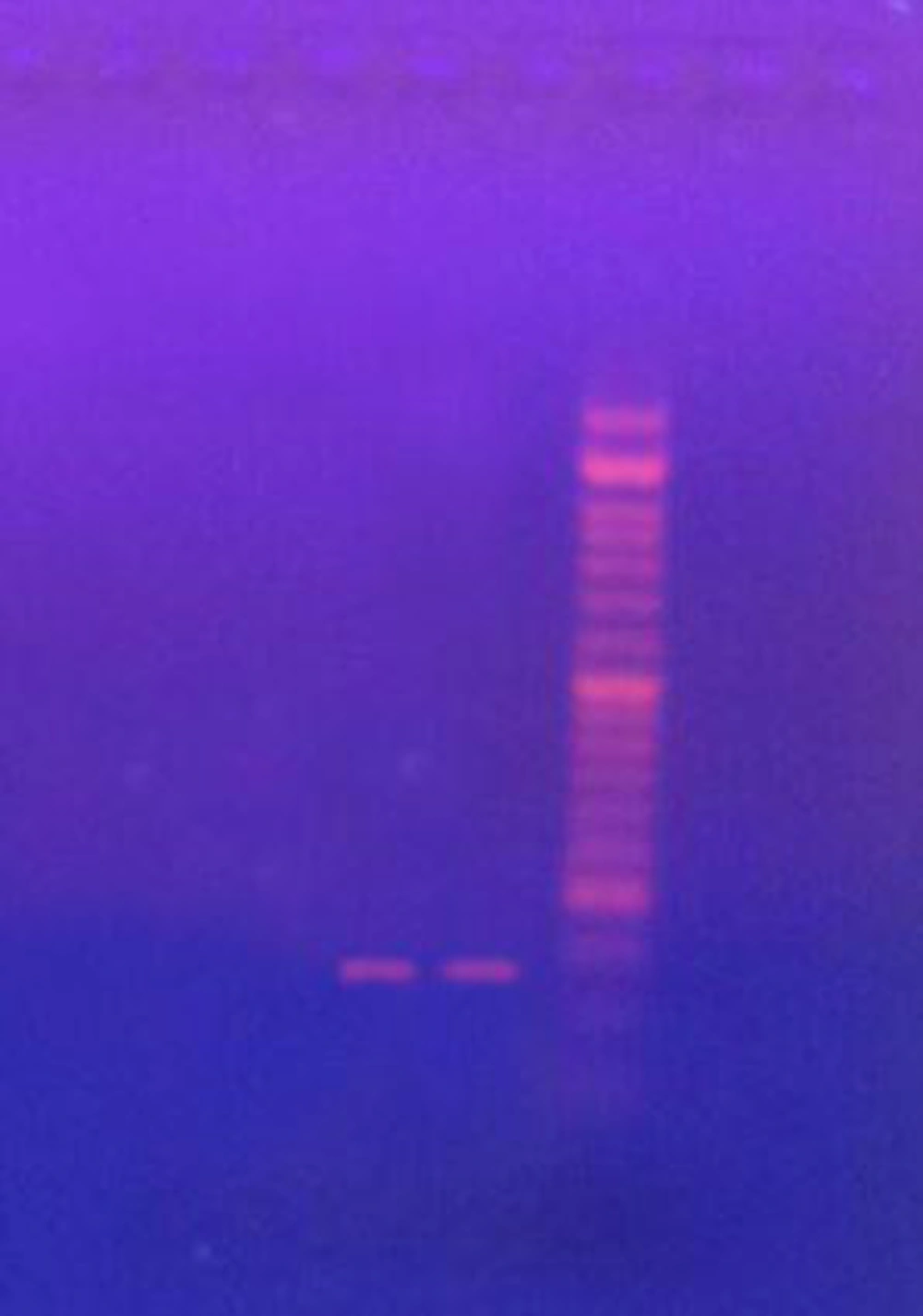
Genomic DNA of C. albicans separate grown nightly in SAB broth was evoke apply a QIAGEN Genomic - tip 20/G and DNA buffer set (Qiagen, Valencia, CA, USA) in accordance with the manufacturer’s instructions, and was applied as a template for amplification of ERG11 (ERG11/ R: 5/ TTTGGTGGTGGAGACATA 3/ , ERG11/ R: 5/ GAACTATAATCAGGGTCAGG - 3/) PCR was perform with high - fidelity 2 DNA polymerase (Roche Molecular Biochemicals, Indianapolis, IN, USA) and sequence - specific oligonucleotide primers . PCR cycling situation were 1 cycle at 95°C for 5 min, pursue by 25 cycles at 95°C for 30 s, 60 °C for 30 s, 72°C for 45 s, pursue by 1 cycle at 72°C for 7 min.
4. Results
The results of this study showed that the highest inhibitory concentration of ethanol extract of T. polium was 150 ppm, while the lowest inhibitory concentration was 25 ppm. The highest fungicidal concentration of ethanoic extract was 300 ppm. The minimum and maximum inhibitory concentration of methanol extract of T. polium is 25 ppm and 100 ppm respectively, of which 1 and 3 strains are inhibited at these concentrations and the highest fungicidal concentration is 200 ppm. The highest and lowest concentrations of ethyl acetate inhibitors are 50 ppm and 12.5 ppm, which 2 and 1 strain are inhibited at these concentrations (Table 1). The results of the study showed that the minimum and maximum concentrations of C. colocynthis ethanoic extract were 12.5 ppm and 100 ppm, of which, 1 and 2 strains were inhibited at these concentrations. The lowest and highest inhibitory concentrations of the ethyl acetate extract were 6.25 ppm and 50 ppm, and also the highest fungicidal concentration was 100 ppm. Our studies display that of all tested race, 65.3% was resisting to nystatin and 73.3% to fluconazole. Of the 7 race, 2 were strain has gene (Figure 1).
| C. Albicans | MIC/MFC | |||||
|---|---|---|---|---|---|---|
| T. polium | C. colocynthis | |||||
| Ethanol | Methanol | Ethyl Acetate | Ethanol | Methanol | Ethyl Acetate | |
| 1 | 100/200 | 100/200 | 50/50 | 50/100 | 50/50 | 25/50 |
| 2 | 150/300 | 100/200 | 50/50 | 25/25 | 50/100 | 12.5/25 |
| 3 | 100/200 | 100/100 | 25/50 | 50/100 | 25/50 | 25/25 |
| 4 | 50/100 | 25/50 | 25/25 | 100/200 | 25/50 | 25/50 |
| 5 | 100/200 | 50/100 | 25/25 | 50/100 | 100/200 | 50/100 |
| 6 | 25/50 | 50/100 | 12.5/25 | 12.5/25 | 25/50 | 6.25/12.5 |
| 7 | 50/100 | 50/100 | 25/50 | 100/200 | 50/100 | 25/50 |
5. Discussion
In recent decades opportunistic infections caused by fungi such as Candida yeast have increased dramatically and therefore, caused the attention of researchers to search in relation to new antifungal drugs, especially paid medicinal plants.
In the study of Zerroug et al., who investigated the antimicrobial activity of T. polium extract against E.coli, B. subtilis, P. diminutus, Paracoccus paratrophus, and Micrococcus luteus, the results showed that the inhibition zone against B. subtilis, M. luteus and P. paratrophus bacteria was 3.7, 2 and 2 mm, respectively (13).
In the study of Shahba, the aqueous, ethanoic and ethyl acetate juice of T. polium herb were prepared. The outcome displayed that T. polium juice were merely efficient in enterococcus and pseudomonas microbial. In general, the MIC price of aqueous juice in enterococcus was 1.25 - 5 mg/mL. The MIC value of ethanoic juice for enterococcus was calculated as 10 mg/mL. The MIC of aqueous and ethyl acetate juice for Pseudomonas bacteria were 5 and 20 mg/mL, respectively. The MBC context of aqueous and ethyl acetate juice of Teucrium for Pseudomonas microbial was 10 mg/mL in aqueous and 20 mg/mL in ethyl acetate juice. The MBC value of juice for enterococcus microbial were 10 mg/mL in aqueous juice and 20 mg/mL in ethanoic juice (14).
In an investigation carried out by Esmaeili et al., the results of the antimicrobial effects of T. polium showed considerable inhibitory effects of this plant on most gram-positive and gram - negative bacteria, which was more effective than antibiotic, as the mean of inhibition zone diameter of Staphylococcus epidermidis, E. coli, and Salmonella were 32, 30, 35 mm, respectively. However, in our study, E. coli samples were resistant to all 3 (aqueous, ethanoic, ethyl acetate) extracts (15). In another research, Darabpour et al., studied the antimicrobial effect of ethanoic and methanol extracts of leaves of the plant on pathogenic bacteria (B. anthracis, B. cereous, S. aureus, S. epidermis, Yersinia enterocolitica, E. coli, Salmonella typhimurium, Bordetella bronchiseptica, Proteus mirabilis, and Antinomyces pyogenes). The results obtained from the ethanoic extract indicated that Staphylococcus epidermis is the most sensitive bacteria and Salmonella typhimurium, Staphylococcus aureus and E. coli bacteria are the most resistant species to this juice. In our study, staphylococcus and E. coli also showed resistance to all 3 types of (aqueous, ethanoic and ethyl acetate) juice (16).
The study of minimal inhibitory concentration of the taken juice compared to the strains resistant to Klebsiella pneumonia showed that all the above extracts have antimicrobial properties and were able to prevent the development of strains resistant to Klebsiellapneumonia, whereas all 8 - strain Klebsiella in the study were resistant to the 3 (aqueous, ethanoic, ethyl acetate) extracts (17).
In a study by Rodge et al., which investigated the antimicrobial activity of C. colocynthis against E. coli, S. aureus, Shigella, C.albicans bacteria, the results showed that estrogens, methanol, ethanol, and water extracts are inhibitors of bacteria (18).
Memon et al., showed that C.colocynthis ethanol extract is a potent inhibitor of Gram - positive bacteria, B. pumilus and S.aureus and gram - negative bacteria, and E.coli and P. aeruginosa. Methanolic, ethanolic, and acetonic extracts of C. colocynthis showed a good inhibitory effect on E.coli (19).
Our studies demonstrated that of all experiment strains, 65.3% were resistant to nystatin and 73.3% to fluconazole. In the study of Saranya, antifungal capacity experimental was done for 26 Candida albicans isolates by the Disc Diffusion Method. The organisms showed complete resistor to Itraconazole 26 separate (100%), Clotrimazole 26 (100%), Nystatin 24 (92.31%), Fluconazole 21 (80.77%), and Keto conazole 17 (65.38%). The organisms demonstrate highly susceptible alone to Amphotericin B 23 (88.46%) (20). The study of Maroszynska, demonstrated that of all experiment races, 7% were resisting to nystatin, 32% to fluconazole, 23% to voriconazole, and no race amplify in the attendance of caspofungin (21).
In the study of Henry et al., Azole - dependent upregulation was not limited to ERG11: 5 of 5 ERG genes experimental whose yield action upstream and downstream of lanosterol demethylase in the sterol biosynthetic transmission were as well as upregulated (22).
The Xiang study examined the prevalence of ERG11 gene in 23 clinical samples of Candida albicans. We observed that substitutions A114S, Y132H, Y132F, K143R, Y257H, and a new K143Q substitution contributed to significant increases (≥ fourfold) in fluconazole and voriconazole resistance; changes in itraconazole resistance were not significant (23).
In the study of Xu et al., the prevalence of ERG11 gene in 23 samples (8 susceptible and 15 resistant) of Candida albicans was investigated.
A total of 19 missense mutations were discovered. of them, 2 mutations, G487T (A114S) and T916C (Y257H), coexisted solely in 14 fluconazole - resistant separate. To know the resisting device in the separate with G487T and T916C mutations, we contrast the explanation of 5 resisting - relevant genes in the 14 azole - resisting separate with those in the sensitive kind race ATCC 10231, Saccharomyces cerevisiae AD/CDR1, and AD/CDR2 (24). The results of this study indicated that extracts of T. polium and C. colocynthis, especially C. colocynthis extract, collected from Sistan region, showed good antimicrobial activity against Candida albicans.