1. Introduction
Visceral leishmaniosis is one of largest parasitic killers in the world, which is annually responsible for more than 200,000 infections worldwide (1). Fever, massive hepatomegaly, and splenomegaly are the most typical symptoms of the disease. The disease usually appears two to six months following infection (2). Without proper treatment the mortality rate for kala-azar is close to 100% (3).
Less invasive diagnostic methods are demonstration of specific antibodies, antigens, or parasite DNA in peripheral blood specimens (4). The direct agglutination test (DAT) has high sensitivity but relatively low specificity (5). The recombinant kinesin antigen (rK39) is a useful antigen in enzyme-linked immunosorbent assay (ELISA), also available in strip format as a rapid test (6). The direct agglutination test (DAT) requires less equipment than ELISA, thus, it is useful in developing settings (7).
The gold standard for the diagnosis of visceral leishmaniosis is the demonstration of parasite in tissue (usually bone marrow or spleen) (8). Bone marrow aspirates are generally safer than splenic aspirates and the sensitivity of bone marrow smear is about 60 to 85%.
2. Case Presentation
Our patient was a 5-year-old girl admitted due to prolonged fever, weight loss and weakness since 1 month ago. Physical examination showed massive hepatosplenomegaly and icter. After admission, laboratory tests revealed the following: red blood cells (RBCs), 3.66 × 109 per Liter; hemoglobin, 8 g/L; white blood cells (WBCs), 3.2 × 109 per Liter with 36% neutrophils, 54% lymphocytes, 6% monocytes, 2% eosinophils, and 1% basophils. A peripheral blood smear showed hypochromic-microcytic anemia. Blood chemistry analysis demonstrated total bilirubin of 4 mg/dL and direct bilirubin of 0.5 mg/dL. Aspartate transaminase (AST) was 134 (0 - 40) and alanine aminotransferase (ALT) 97 (0 - 55), and virology tests were negative. Chest X-ray was NL. Abdominal sonography showed only massive hepatosplenomegaly, and rapid ELISA test was negative for leishmaniosis.
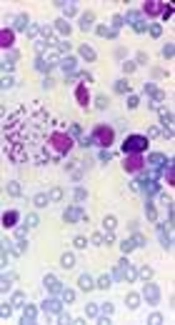
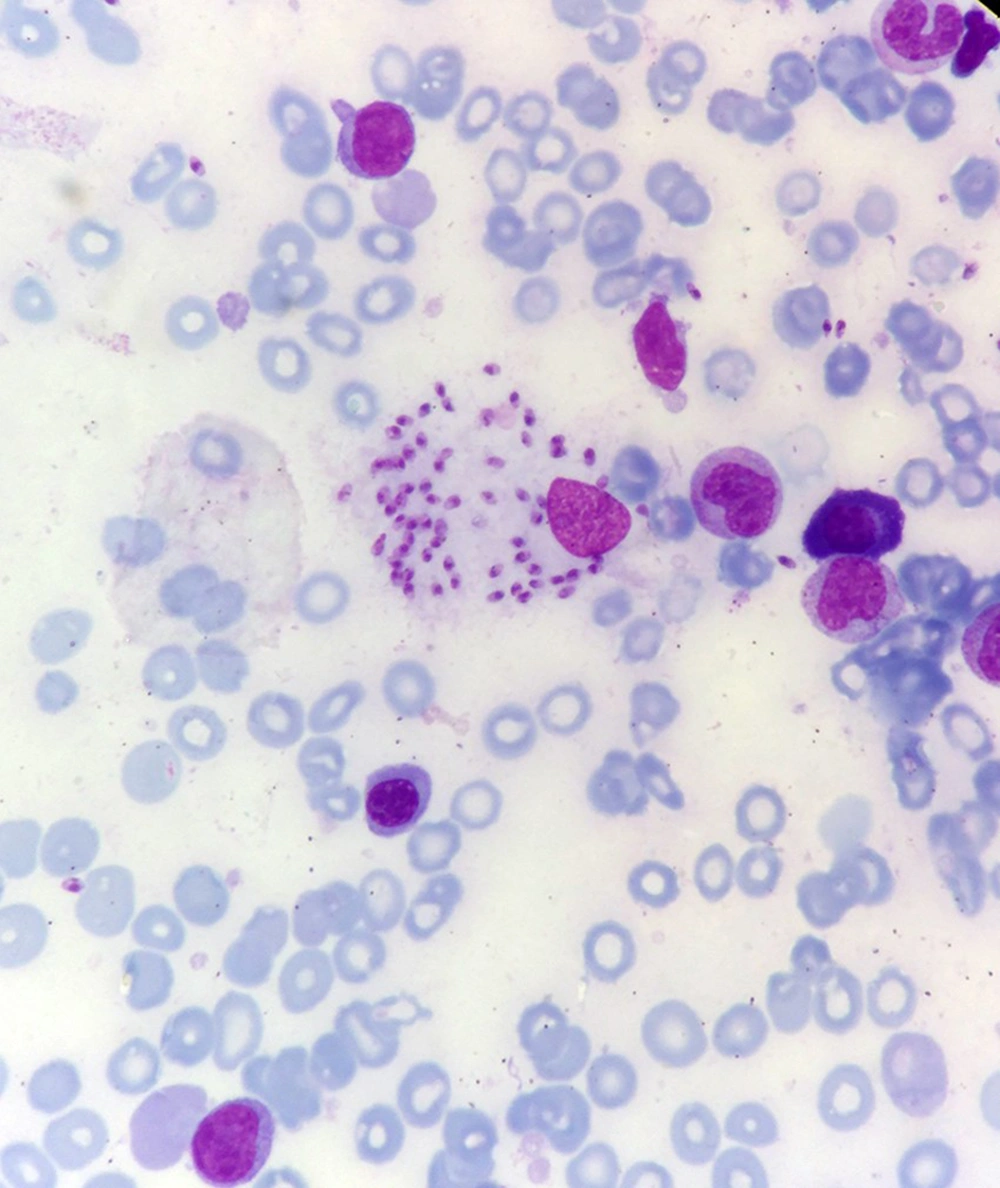
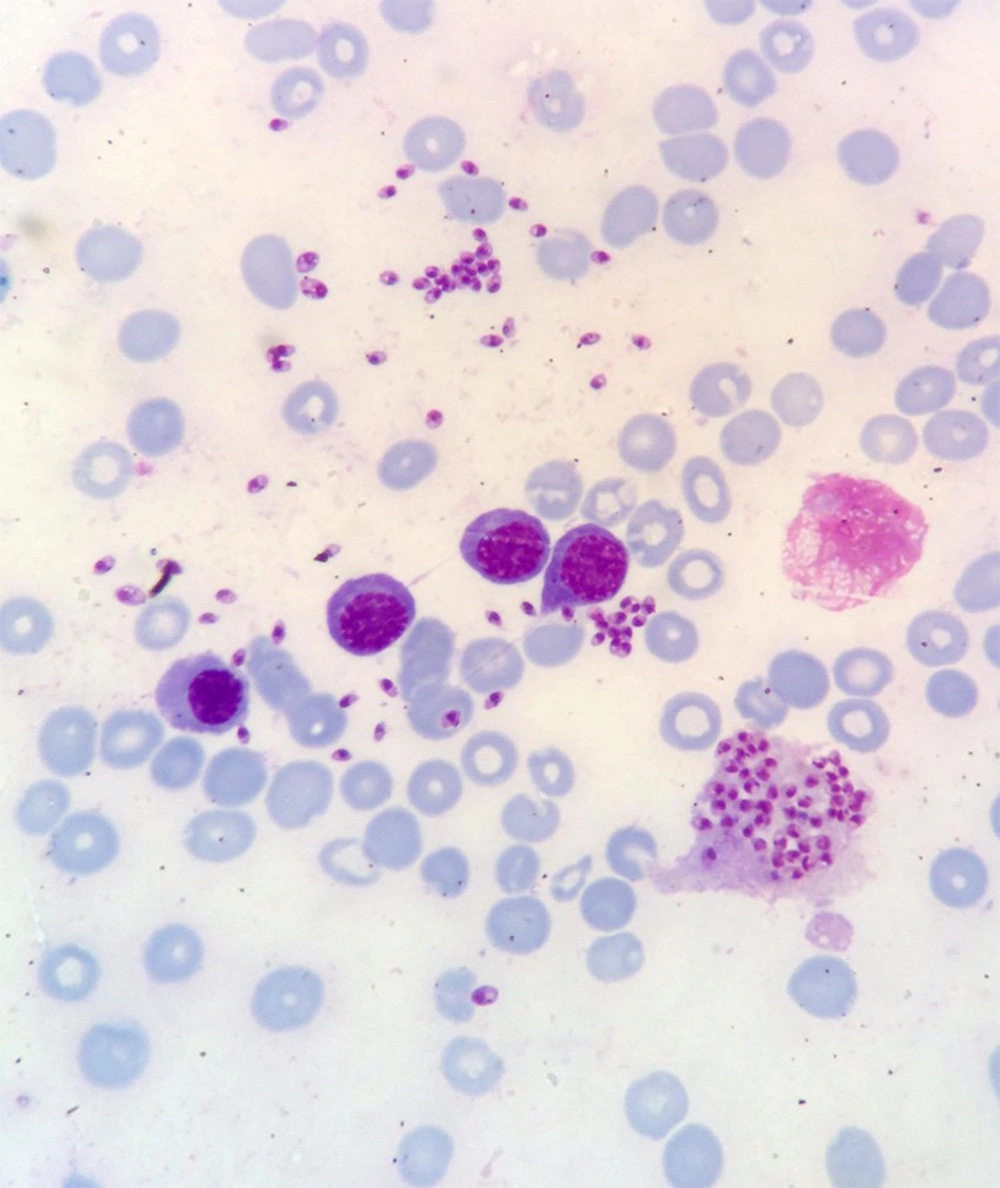
Bone marrow aspiration and bone marrow biopsy were performed, showing spherical or ovoid bodies, amastigotes, within macrophages and outside cells (Figures 1 and 2).
3. Discussion
Although there are new diagnostic tools for visceral leishmaniosis, but the gold standard is visualization of the amastigotes in splenic or bone marrow aspirate. Montenegro skin test, a Leishman skin test, is negative in active visceral leishmaniosis and is no longer used (9).
The direct agglutination test (DAT), rK39 in ELISA, and indirect fluorescent antibody test (IFA) are not definitive proof of active visceral leishmaniosis, and in endemic areas positive antibody tests may be observed among asymptomatic individuals with subclinical infection (6, 10), and up to 32% of the healthy population may have a positive test but do not require treatment (11).
In our case, ELISA test was negative, and as noted in Figures 1 and 2, bone marrow aspiration showed Leishman bodies inside and outside macrophages in Wright-Giemsa stain.