1. Background
Gastric infection with H. pylori is responsible for most cases of peptic ulcer disease, gastric adenocarcinoma, and gastric MALToma (1-3). Therefore, eradication of the infection will prevent many of these complications (3). On the other hand, H. pylori infection is present in nearly 90% of the adult population and appears to occur early in life, and more than 50% of children are infected before age 15 (3, 4). There are different regimens for the first-line treatment of the infection, and the eradication rates of most successful regimens range from 75% to 92% (4). Epidemiological studies in Iran have revealed the prevalence of H. pylori infection in patients over 35 years old is about 86%; however, according to clinical studies conducted in Iran, the rate of eradication of the infection is less than the reported level in western and some developing countries (5, 6).
Many researchers have reported high levels of resistance to metronidazole (37%) and clarithromycin (28%) (2, 3, 7-11). Fakheri et al. (12), in a study, showed that furazolidone-based quadruple therapy is a good alternative for eradication of H. pylori infection in regions with high resistance to metronidazole and clarithromycin. Based on an academic standpoint, the best regimen for eradication of H. pylori infection should be selected by regional studies (1, 2, 10, 12) but such recommendation, in practice, is difficult.
2. Objectives
In this study, we compared two regimens that widely were used in Iran, triple regimen (clarithromycin, amoxicillin, and omeprazole) and furazolidone-based quadruple regimen (furazolidone, amoxicillin, bismuth, and omeprazole), in some dyspeptic patients who were infected with H. pylori.
3. Methods
This cross-sectional study was conducted on 18 years older patients who were referred to gastroenterology clinic of Ali-ebn-Abitaleb teaching hospital in Zahedan, suffering from dyspepsia and had confirmed H. pylori infection using Urea Breath test (UBT) or Rapid Urease test (RUT). Pregnant women, patients with a history of chronic kidney, liver, and lung diseases, malignancy, G6PD deficiency, gastrointestinal (GI) surgery, GI bleeding, history of taking non-steroidal anti-inflammatory drugs (NSAIDs), taking antibiotic during recent 4 weeks, anti-H. pylori treatment during recent years and a history of known drug sensitivity to each of the study drugs were excluded from the study. Our main outcome was H. pylori eradication as established by a negative UBT at least four weeks after the end of the treatment.
Eligible patients were randomly divided into one of the following groups; group A (treated with clarithromycin 500 mg, amoxicillin 1,000 mg, and omeprazole, 20 mg twice daily for 10 days) or group B (furazolidone 200 mg, amoxicillin 1,000 mg, bismuth subcitrate 240 mg, and omeprazole 20 mg twice daily for 14 days). Patients were evaluated for eradication of H. pylori infection using the respiratory13C-urea test four weeks after the treatment. All of the patients were instructed about the correct use of the drugs and probable adverse effects by a face to face interview and a leaflet, containing all recommendation about the medications and their side effects. Moreover, they were advised to call us in the event of any adverse effect(s) without consumption stops. We also, every week, checked the patients’ compliance by pill counting and recorded any adverse events and, with patient assurance, encourage them to drug consumption. In a newly started medication for other propose(s), if medication had potential interaction with the study medications, the patient was excluded from the study. If the patient consumed continuously (without any disruption or gap at consumption) at least 90 percent of the given regimen, it was considered acceptable compliance. The project was approved by the local Ethics Committee of Zahedan University of Medical Sciences, and written informed consent was obtained from each individual participating in this study. The collected data were analyzed by SPSS ver. 18, and for statistical analysis, we used chi-square and student t-test, and a P-value of less than 0.05 was considered statistically significant.
4. Results
Among 512 dyspeptic patients, 373 people were eligible to enter the study who were randomly divided into one of the two groups, 188 in group A and 185 patients in B. Of these patients, five (0.25 %) and seven (0.29 %) patients of group A and B were dropped, respectively, due to consent withdrawal or not returning for UBT, and finally, 183 patients in group A and 178 patients in group B completed the course of the project. The patients were between 18-59 years old with an average age of 35.9 ± 12.3 and male to female ratio of 1:1.63. The demographic characteristics of the patients and their response rate to each regimen are summarized in Table 1.
| Group | No (M/F) | Mean Age ± SD | Eradication, No. (%) | Non-eradication, No. (%) | ||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | |||
| Triple-drug regimen | 183 (65/118) | 36 ± 12.7 | 44 (68) | 73 (62) | 21(32) | 45 (38) |
| Quadruple-drug regimen | 178 (74/104) | 35.7 ± 11.9 | 71 (96) | 95 (91) | 3 (4) | 9 (9) |
| P-value | 0.787 | 0.152 | 0.115 | 0.341 | ||
Abbreviations: F, female; M, male; N, number; SD, standard deviation.
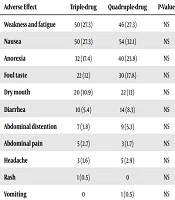
The mean age of the patients of group A who responded to the treatment was not statistically different from those who did not. However, patients that did not respond to the treatment had statistically significant older mean age in group B (P = 0.001). Regarding side effects of the regimen, there was no statistically significant difference between the two groups. The frequencies of adverse effects in the two groups are summarized in Table 2.
| Adverse Effect | Triple-Drug | Quadruple-Drug | P-Value |
|---|---|---|---|
| Weakness and fatigue | 50 (27.3) | 46 (27.3) | NS |
| Nausea | 50 (27.3) | 54 (32.1) | NS |
| Anorexia | 32 (17.4) | 40 (23.8) | NS |
| Foul taste | 22 (12) | 30 (17.8) | NS |
| Dry mouth | 20 (10.9) | 22 (13) | NS |
| Diarrhea | 10 (5.4) | 14 (8.3) | NS |
| Abdominal distention | 7 (3.8) | 9 (5.3) | NS |
| Abdominal pain | 5 (2.7) | 3 (1.7) | NS |
| Headache | 3 (1.6) | 5 (2.9) | NS |
| Rash | 1 (0.5) | 0 | NS |
| Vomiting | 0 | 1 (0.5) | NS |
aValues are expressed as No. (%).
5. Discussion
Just three to four decades after the identification of H. pylori and multiple regimens that were used for eradication of this microbe in human, now it appears that a considerable number of infected patients have no response to some of the eradication regimens; thus, most authors advised performing culture and antibiogram sensitivity. However, in practice, especially in low-income countries, this academic recommendation is often difficult and occasionally impossible. In clinical practice, the eligible rate of eradication of H. pylori infection according to the intention-to-treat studies is 85% to 90%. Our study showed that triple-clarithromycin based and probably most prescribed H. pylori- eradicating regimens (2, 3, 13, 14), provide inferior results compared with antimicrobial therapies for other common infectious diseases, and furazolidone-based quadruple regimen is superior to standard triple regimen; thus it is recommended with greater confidence.
Studies have demonstrated an increasing number of patients with failure to respond well to the treatment due to antibiotic resistance in recent years. In two decades ago, triple-drug regimens, including a proton pump inhibitor (PPI) and two antibiotics, as the first-line treatment for H. pylori resulted in an 80% eradication rate in the world, and now, probably is effective in some regions (3, 14-17). However, according to some studies in Iran, about 37.5% of H. pylori strains are resistant to metronidazole, 28% to clarithromycin, and among 140 isolates tested for susceptibility to furazolidone, seven (5) were resistant.
In addition, some studies concluded that furazolidone-based regimens are superior to metronidazole-based treatments for Iranian patients, (per-protocol eradication rate of 95.2% in furazolidone-based group Vs 83.1% in the metronidazole-based group). For these reasons, it seems that the most effective first-line treatment is quadruple therapy with furazolidone for 14 days. The rate of eradication in the furazolidone-based branch in the current study is similar to the other researches in our country (9). Therefore, it is better to encourage practitioners for its prescription. However, treatment-associated side effects were more common in the furazolidone-based regimen (4, 5, 12).
Although we did not perform culture and antibiotic sensitivity, the low efficacy of the triple-based regimen in the present study is probably due to resistance to clarithromycin. Clarithromycin is not only expensive but also has GI adverse effects and must be overlooked for H. pylori eradication regimen, at least in low-income countries (6, 7). Rimbara et al. (16) in a review in 2011 recommended practitioners to avoid clarithromycin-based triple therapy. The rate of eradication of H. pylori infection by furazolidone-based regimen was reported to be approximately 80% in various studies (1, 6, 9). Furazolidone, a nitrofuran derivative with bacteriostatic or bactericidal properties against both Gram-negative and Gram-positive bacteria, is absorbed well from the intestinal wall and has no tissue concentration, and since relatively low resistance strains of H. pylori to furazolidone has been reported yet, it could be administered for the treatment of H. pylori infection instead of metronidazole.
One of the major drawbacks of furazolidone is GI intolerance due to bloating, which may result in discontinuation of therapy by some patients and therefore, its use is limited (8, 12, 13). However, side effects are reduced by lowering the dose. Some researchers selected a dose of 100 mg of furazolidone twice a day to reduce the rate of adverse effects meanwhile increase patients’ compliance. According to these studies, when a low dose of furazolidone (100 mg B.D.) is used, does not yield acceptable success rates. Researchers have reported that the rate of eradication of furazolidone-based quadruple drug regimen was 54% and 72% based on intention-to-treat and per-protocol analysis, respectively (12). accordingly, if furazolidone dose was increased to 200 mg two times per day, eradication rate might dramatically improve. Patient adherence is essential for the successful eradication of H. pylori. Given the high pill burden, the increased frequency of administration, and the prolonged duration of treatment, a thorough understanding of the importance of completing the treatment regimen, as prescribed, is paramount, and we can improve our patients’ compliance and H. pylori eradication rate by encouraging and assuring patients. However, choosing the treatment should be based on regional patterns of drug susceptibility and resistance and other factors such as a history of recent taking antibiotics (2, 11, 14, 15, 17-19).
One of the positive points of the current study is high patient number than previous studies, and it was done almost ten years after previous research, and our findings showed that furazolidone-based quadruple therapy is still superior to clarithromycin-based regimen. But one of the weak points of our research was the absence of culture and antibiogram for H. pylori sensitivity. Furthermore, it was better to perform UBT six months after completion of the treatment for recrudescence of H. pylori infection and absolute H. pylori eradication.
In conclusion, because the eradication of H. pylori plays a critical role in the treatment of peptic ulcer disease, MALToma and probably gastric adenocarcinoma prevention, we recommend furazolidone-based quadruple therapy with furazolidone 200 mg twice a day.