1. Background
Healthcare-associated infections are nowadays a real public health problem with a significant socioeconomic burden on patients (1). They are of particular concern because they combine antimicrobial resistance, which is also a significant threat to public health (2). This dual tandem is a major concern in health care facilities in both developing and developed countries (1).
Multidrug-resistant organisms have the particularity of being in a hospital environment because of not only the intensive use of antibiotics for the treatment of patients but also the presence of antibiotic residues in liquid effluents and patients (3). Added to this are invasive medical procedures and devices and non-compliance with hygiene rules that increase the probability of exposure of patients to these germs (4). These organisms can colonize the surfaces of the hospital environment and equipment (5-8). The biological risk is, therefore, very high.
Surgical services and the operating theater are particularly affected by the biological risk. Superinfection of wounds, for example, is of particular concern for public health because of the risk of serious bacterial infections, including gas gangrene and tetanus, which can lead to long-term disability, chronic wound infection, bone infection, or even death. The presence of an injury in a care facility is, therefore, a risk for the patient because of the presence of multidrug-resistant germs, invasive gestures, and equipment.
Faced with this urgency, which is due to the multi-resistant nature of the bacteria involved in nosocomial infections, standard precautions represent the first barrier measures to be implemented and constitute the basic strategy for the prevention of cross-transmission of microorganisms (9-11). In developing countries such as Benin, many factors can hinder compliance with standard precautions. These include the lack of training of health care personnel (12) and inadequate resources for good application of standard precautions. In support of standard precautions, an infection risk assessment of health services is necessary to assess the probability of emergence and the incidence of multidrug-resistant bacteria proliferation in the hospital environment. Studies carried out in the reference university hospitals have shown a real risk linked to the presence of multidrug-resistant bacteria in health care facilities. We can cite, among others, the studies implemented by Degbey et al. (13), and Degbey et al. (14), and Ouendo et al. (15), carried out in the departments of the Center National Hospitalier et Universitaire HKM de Cotonou. These studies have shown poor knowledge and poor application of standard precautions in environments heavily contaminated with multidrug-resistant bacteria. A study by Dougnon et al. (16) showed the contamination of the operative wounds of women with cesarean sections by multidrug-resistant bacteria coming from the environment of the sampling room of the University Hospital of the Mother and Child-Lagoon of Cotonou. The involvement of invasive procedures in the occurrence of bacteremia linked to catheters was also noted by this study. Similar results have been noted for secondary hospitals such as the secondary hospital of Abomey-Calavi/So-ava (17).
2. Objectives
In this context, this study was initiated to evaluate the antimicrobial resistance and infection risks in Tertiary hospitals in Benin. The tertiary hospitals of Sakété-Ifangni and Menontin were chosen as the study cases.
3. Methods
3.1. Sampling
The samples were collected by swabbing the hospital environment (surgery equipment, door keys, mattresses, operating beds, benches, staff, and surgical instruments) and wounds according to the methodology described by Bankole et al. (18). The swabbing of the hospital environment was done in both hospitals (tertiary hospitals of Sakété-Ifangni and Menontin). Wound samples were only collected from the Tertiary Hospital of Menontin because during the study, there were a lot of injuries in this hospital. The collected samples were kept free and taken immediately to the laboratory for bacteriological analyses. The collected swabs were put in coolers with cold accumulators (4°C) before being transferred to the laboratory (maximum 30 min).
3.2. Bacteriological Examination
The collected samples were examined bacteriologically according to conventional methods. We spilled 2 mL of sterile broth on swabs. The suspensions were incubated at 37°C for 18 h. These suspensions were then inoculated on Mueller Hinton agar and Mannitol salt agar, followed by incubation at 37°C for 24 h. Each type of colony was transplanted to obtain a pure strain, followed by microscopic examination (wet preparation and Gram staining). Biochemical tests were performed according to the results obtained in Gram staining. For Gram-negative bacilli, the performed tests were oxidase test, glucose fermentation test, and realization of the API 20E gallery for Enterobacteriaceae differentiation. Biochemical tests for the identification of Gram-positive cohorts were catalase, free staphylocoagulase, and DNase production (16, 18).
3.3. Antibiotic Susceptibility Test
Depending on the bacterial species identified, two panels of antibiotics were used to test the susceptibility of the strains to antibiotics. We followed the Kirby-Bauer method (19), which consisted of the diffusion of antibiotic discs on Mueller Hinton agar according to the recommendations of the French Society of Microbiology. The inhibition zone diameters were compared to the inhibition diameters proposed by the CA-SFM (20). The choice of antibiotic discs was inspired by the recommendations of the French Society for Microbiology and conformed to those used by Dougnon et al. (16).
Antibiotics used for susceptibility testing of cocci strains included penicillin (P), oxacillin (OX), cefoxitin (FOX), clindamycin (DA), erythromycin (E) (15 µg), pristinamycin (PT) (15 µg), and oxacillin (5 µg). Antibiotics used for susceptibility testing of bacilli strains included amoxicillin (AX), clavulanic acid + amoxicillin (AMC), ceftriaxone (CRO), aztreonam (ATM), and imipenem (IPM).
4. Results
4.1. Biological Risks at Sakété-Ifangni Hospital
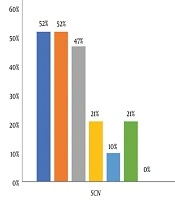
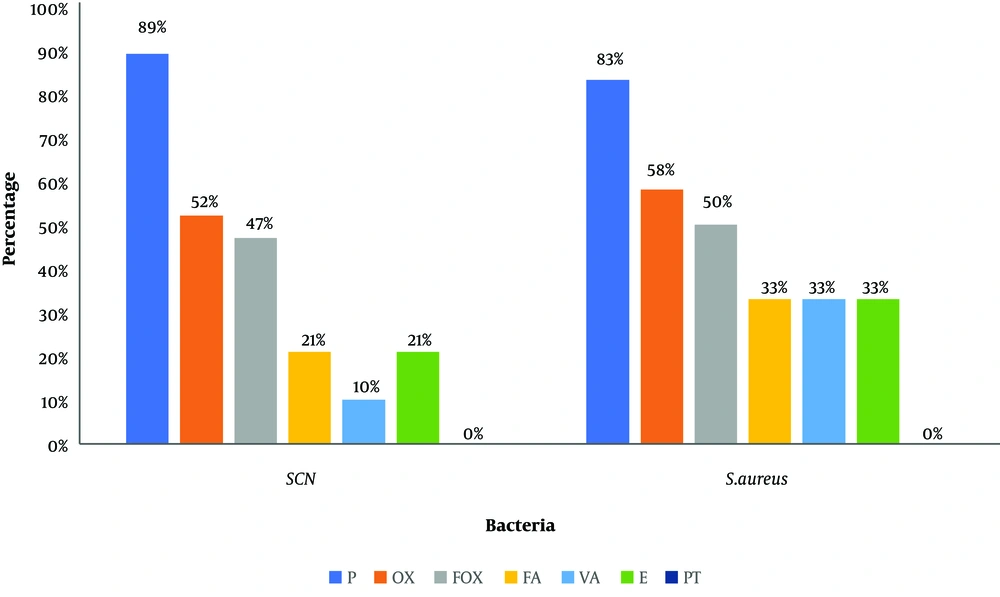
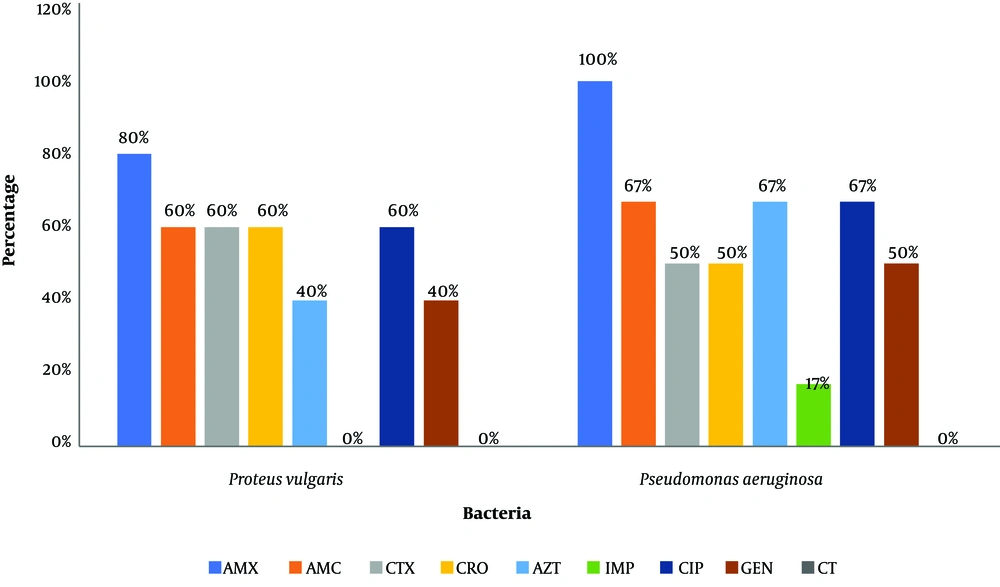
The bacteriological examination of swabs from surfaces and instruments revealed the presence of germs in all samples. Thus, 42 bacterial strains were isolated from 35 samples. The distribution of the isolated bacteria is as follows: coagulase-negative staphylococci (SCNs) (45%), Staphylococcus aureus (27.5%), Pseudomonas aeruginosa (15%), and Proteus spp. (12.5%). Figures 1 and 2 present the resistance profile of cocci and bacilli strains, respectively, isolated from Sakété-Ifangni hospital.
Resistance profile of bacilli strains isolated from Sakété-Ifangni hospital. AMX: amoxicillin, 25 μg; AMC: amoxicillin + clavulanic acid, 30 μg; CTX: cefotaxime; CRO: ceftriazone, 30 μg; ATM: aztreonam; IMP: imipenem, 10 μg; CIP: ciprofloxacin, 5 μg, GEN: gentamycin, 15 μg; CT: colistin, 50 μg.
4.2. Biological Risk at Menontin Hospital
We collected 105 samples from Menontin Hospital, consisting of 60 wound swabs and 45 environmental swabs. After the bacteriological examination, only one sample was sterile (2%) while 75% of the samples were infected by two strains from wound swabs. For environmental swabs, 29 out of 45 were positive and 41 bacterial strains were isolated.
The study of the sociodemographic data of the wounded showed that there were 50% women and 50% men in the sample. The study of the causes of wounds showed that the majority of the patients (57%) had wounds due to injuries, followed by cesarean (23%), burns (2%) and others surgery (18%).
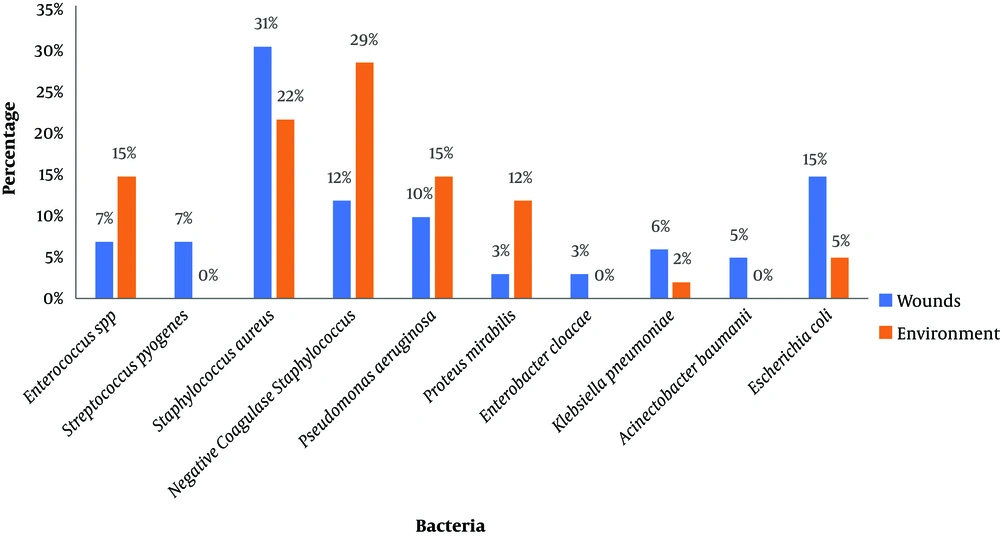
Staphylococcus aureus (31%), Escherichia coli (15%), and coagulase-negative staphylococci (12%) were the predominant species isolated from wounds. Strains of coagulase-negative staphylococci (29%), Pseudomonas aeruginosa (15%), and Proteus mirabilis (12%) were the most common strains in swabs from room environments (Figure 3).
Antibiotic susceptibility revealed that Gram-negative bacilli had high beta-lactam resistance levels, except to imipenem for which low resistance was noted only for Escherichia coli strains, Pseudomonas aeruginosa, and Acinetobacter spp. (Table 1). Many strains isolated from the room environment were found in the wounds of patients, as well.
| AX | AMC | CRO | ATM | IPM | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| W | E | W | E | W | E | W | E | W | E | |
| Escherichia coli | 95 | 100 | 82 | 50 | 82 | 50 | 91 | 50 | 9 | 0 |
| Klebsiella pneumoniae | 100 | 100 | 66 | 100 | 66 | 100 | 33 | 100 | 0 | 0 |
| Enterobacter cloacae | 100 | - | 100 | - | 33 | - | 33 | - | 0 | - |
| Proteus mirabilis | 88 | 80 | 66 | 60 | 33 | 60 | 33 | 60 | 0 | 0 |
| Pseudomonasaeruginosa | 92 | 83 | 75 | 67 | 62 | 67 | 50 | 67 | 25 | 17 |
| Acinetobacter spp. | 82 | - | 70 | - | 60 | - | 60 | - | 16 | - |
Abbreviations: AMC, clavulanic acid + amoxicillin; ATM, aztreonam; AX, amoxicillin; CRO = ceftriaxone, E, environment; IPM, imipenem; W, wound.
For Gram-positive cocci strains, there was no evidence of resistance to vancomycin, except for Staphylococcus aureus where 8% of the cases of resistance were noted (Table 2).
| P | OX | FOX | VAN | DA | E | PT | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| W | En | W | En | W | En | W | En | W | En | W | En | W | En | |
| S. aureus | 60 | 80 | 60 | 80 | 48 | 65 | 8 | 12 | 0 | 0 | 12 | 22 | 0 | 0 |
| SCN | 89 | 72 | 89 | 72 | 52 | 80 | 0 | 0 | 0 | 0 | 15 | 28 | 0 | 0 |
| Enterococcus spp. | 100 | 100 | 100 | 100 | 100 | 100 | 0 | 0 | 70 | 100 | 100 | 100 | 0 | 0 |
| S. pyogenes | 70 | - | 70 | - | 100 | - | 0 | - | 80 | - | 100 | - | 0 | - |
Abbreviations: DA, clindamycin; E, erythromycin 15 µg; En, environment; FOX, cefoxitin; OX, oxacillin; P, penicillin; PT, pristinamycin 15 µg, Oxacillin 5 µg; S. aureus, Staphylococcus aureus; S. pyogenes, Streptococcus pyogenes; VAN, vancomycin 30 µg; W, wounds.
The study of the adequacy of treatment of infections due to the bacteria responsible for wound suppuration showed that for 38% of the wounded, no antibiotic treatment was used (just antiseptics were used). For 52% of the wounded, the antibiotic treatment was in line with the results of the antibiogram. However, 10% of the wounded were not receiving antibiotic treatment in line with the results of the antibiogram.
5. Discussion
This study aimed to evaluate the antimicrobial resistance and infection risks in Tertiary hospitals in Benin. The tertiaries hospitals of Sakété-Ifangni and Menontin were chosen as the study cases. The evaluation of the infection risk at Sakété-Ifangni hospital showed the presence of SCN (45%) as the predominant species, followed by Staphylococcus aureus (27.50%) and Pseudomonas aeruginosa (15%). Many other studies highlighted the role of these pathogens in the contamination of surfaces and nosocomial infections (6, 7, 9). Even if our study agrees with the study by Afle et al. (17), as regards the presence of these germs, they differ in the prevalence of germs found. Indeed, this study revealed the predominance of Staphylococcus aureus, Klebsiella spp., and Acinetobacter baumannii. Klebsiella pneumoniae, Escherichia coli, and Enterobacter cloacae were the predominant germs found in the environment of the National University Hospital Center HKM of Cotonou (15). These discrepancies show the diversity of pathogens involved in contamination according to the hospital center. It would, therefore, be important to establish a map of germs according to health centers in order to better control the spread of multidrug-resistant bacteria responsible for infections associated with healthcare settings. The study of antibiotic resistance showed numerous resistant strains in accordance with the literature (21, 22). The strong presence of SCN is an indicator of poor hand hygiene. The study by Degbey et al. in 2016 (14) already highlighted the poor quality of hand hygiene among health professionals in Benin, but it is well known that hand hygiene alone can reduce 80% of the frequency of infections. It is important that these health professionals adopt good practices in the healthcare setting (23).
Out of 60 festering wounds swabbed at Menontin Hospital, 59 showed the presence of at least one bacterium. Moreover, 75% of wound pus samples contained two bacterial strains. The production of pus by wounds is the most visible sign of colonization of the wounds by bacteria. Therefore, wounds can be contaminated by microorganisms that colonize the surfaces and air. In this hospital, the material used during dressing (pliers, compress, scissors, beans, and trays) was contaminated, which highlights the problem of sterilization and preservation of the sterilized material. These results corroborate those obtained by Degbey et al. in 2013 (13) in a study on the quality of technical medical equipment used in the operating theaters of the Center National Hospitalier Universitaire HKM de Cotonou. A study by Slekovec et al. (24) showed that wound care is also a source of contamination of surfaces and equipment that can spread to subsequent patients. It is, therefore, a source of the spread of infections within hospitals. These hospitals need to incorporate good bio-cleaning and sterilization techniques for technical medical equipment into their programs in order to control the risk of infection in hospitals. Among the bacteria involved in the suppuration of the wounds, we mostly isolated Staphylococcus aureus (31%), followed by Escherichia coli (15%) and coagulase-negative staphylococci (12%). They are the bacteria encountered in nosocomial infections, in general, and in wound infections, in particular (24, 25). The multi-resistant nature of the isolated strains is also in agreement with the hospital origin of the strains because the hospital strains are well known for their multi-resistance (21). The comparison of the resistance profiles showed that there were the same profiles of circulating strains in the environment and wounds. The resistance proportions obtained for bacteria isolated from wounds and the environment in the present study are close to those of bacteria isolated from wounds and the environment at the University Hospital of Mother and Child-Lagoon (16). This study, therefore, confirms the role of the environment, especially the collection rooms, in the colonization of wounds in patients. It also shows that the issue of antimicrobial resistance is the same reality in health centers regardless of levels.
The study of the adequacy of the treatment showed that for 38% of the infected wounds, no antibiotic treatment was administered and 10% of treatment was contrary to the result of the antibiogram. Indeed, the action of antiseptics is limited to the bacteria present during the operation although the good quality of antibiotics is needed. The inappropriate use of antibiotics without screening is likely to increase the incidence of multidrug-resistant bacteria. These results reveal a problem in the prescription of antibiotics in hospitals. Indeed, the prescription of antibiotics requires a good diagnostic approach including the establishment of an etiological diagnosis either by the isolation and identification of the infectious agent or by serological tests to establish contact with the pathogen. Ouedraogo et al. (26). Therefore, before any antibiotic prescription to an injured patient, it is important to carry out bacteriological examinations of wound swabs to provide adequate management of wound superinfections by multidrug-resistant bacteria.
5.1. Limits of the Study
This study was carried out in two Tertiary hospitals in South Benin. As socio-professional realities are not the same from one region to another and from one hospital to another, this study does not reflect the situation in Benin. A more extensive study is needed in other regions and other hospitals for a more comprehensive assessment taking into account all realities. We also suggest that healthcare professionals become aware of the care administered and the antibiotics prescribed. This will reduce infections in the healthcare environment.
5.2. Conclusions
This study revealed a significant biological risk in Tertiary hospitals in Benin. This risk is marked by the presence of pathogenic bacteria in the environment and the wounds of injured patients. This risk is aggravated by the multidrug resistance of circulating strains. It is fundamental to put in place a strategic policy for the treatment of wounds. It is, therefore, important that the necessary means and recommended devices be put in place for better prevention of nosocomial infections and good patient safety.