1. Background
Wolfram syndrome (WS) is a rare hereditary neurodegenerative disease that was first described by Wolfram and Wagener in 1938 (1). This syndrome is characterized by coincidence of early-onset diabetes mellitus and optic atrophy, followed by a wide spectrum of manifestations affecting different organs, including sensorineural deafness, urological abnormalities, psychological abnormalities, and endocrine disorders (2).
WS prevalence is estimated to be around 1 in 68,000 - 770,000, differing based on racial differences (3, 4). This syndrome bears a poor prognosis and yet there is no effective treatment to prevent its progression. The mortality rate in patients under 35 years old is 65% and patients affected by WS have a median life expectancy of 30 years (5).
The first causative genetic mutation was found in WFS1 locus which encodes Wolframin, an endoplasmic reticulum (ER) transmembrane protein involved in membrane trafficking and regulation of ER calcium homeostasis. Wolfram syndrome type 2 is due to mutations in the CISD2 gene that encodes another ER intramembranous protein. Both types of WS are usually inherited in an autosomal recessive pattern (6, 7).
Although urinary tract dysfunctions include upper tract dilatation and bladder dysfunctions are amongst common reported manifestations of WS, their etiology is still unclear but the main hypotheses behind these dysfunctions are distention injuries secondary to polyuria and autonomic dysfunctions (8).
Up0per urinary tract dilatation is an important entity since it increases the risk of urinary tract infections and progression of acute and chronic renal failure. However, controversies exist regarding its etiology as well as its treatment (2, 9).
Because of the difference in treatment approach toward different subclasses of hydroureter in WS and the necessity of surgical intervention for the obstructive types of hydroureter, it is essential to distinguish between obstructive and non-obstructive hydroureters in any affected patient.
2. Objectives
Current established modalities for discriminating obstructive and functional hydroureters are sparse and restricted due to their invasiveness and low specificity. Based on previous studies drainage-related ultrasonography (DRUS) can be an efficient radiological modality in distinction of the hydroureters’ types. In this study, we prospectively studied application of DRUS in WS in detection of hydroureters’ types in order to prevent invasive surgical interventions.
3. Methods
Patients with hydrouretronephrosis on ultrasonography who were previously diagnosed with WS and were hospitalized in Children’s Medical Center, Tehran, from 2010 to 2016 were included in the study. Patients who had accompanying urogenital anomalies, and had undergone surgical repair or had transient hydroureter, were excluded. Moreover, incomplete medical records and incompliance with the study were regarded as exclusion criteria.
Wolfram syndrome diagnosis in all patients was based on following criteria: Insulin-dependent diabetes accompanied by optic nerve atrophy that was not associated with other diseases or abnormalities. The diagnosis was established finally by genetic examination for WFS1 gene mutations.
All patients have been evaluated for urologic manifestations of this syndrome by urinalysis, urine culture, renal function tests (serum creatinine), urinary tract ultrasound, Voiding cystourethrography (VCUG), magnetic resonance urography (MRU) and urodynamic studies.
Patients were closely inspected and underwent routine follow-ups after diagnosis by monthly urinalysis, urine culture and renal function tests. If recurrent UTI was observed in a patient, further investigation was done by urinary tract ultrasound and VCUG, every 3 months and every 6 months, respectively up to one year.
In order to distinguish the etiology of hydrouretronephrosis among patients, Drainage related ultrasonography (DRUS), a recently-introduced technique in ultrasonography, was performed in patients with urinary tract dilations detected in ultrasound, MRU or VCUG. DRUS in all patients was performed by a single pediatric radiologist to avoid operator-dependent variations.
All parents were provided with a detailed description prior to DRUS, and oral informed consent was obtained from all parents or guardians. All participants received care according to the 2008 World Medical Association Declaration of Helsinki.
DRUS technique consists of several steps. Initially a baseline US of upper urinary tract is indispensable to provide us with baseline measurements of renal parenchymal diameter, renal pelvis diameter and ureteral diameters on longitudinal views. To assure the reliability of these measurements and its independence from bladder fullness, toilet-trained children were requested to void prior to continuing evaluation. In case measurements were altered after voiding, the patient was excluded from the study.
Afterwards a three-hours bladder drainage was performed with the aid of ureteral catheterization by proper Nelaton catheter (Figure 1). Patients were not restricted regarding walking, food and drinks during the 3-hours catheterization; they were closely watched by a pediatric radiologist and a pediatric urologist during the process.
The largest diameter of hydroureter was expressed as D1 prior to drainage and D2 after 3h drainage. Renal parenchymal cortical diameter was also expressed as P1 prior to drainage and P2 after 3h drainage. D ratio and P ratio which are demonstrative of rate of changes in ureteral diameter and renal parenchymal diameter respectively, are calculated using following formulas:
The initiative study on DRUS by Kajbafzadeh et al. (10), that introduced DRUS as a technique for discriminating obstructive and non-obstructive hydroureters for the first time, set a cutoff point of 22% for D ratio (Sensitivity = 78.5%, specificity = 83.4%) for differentiating between obstructive and non-obstructive hydroureters.
Based on the calculations and rules that were introduced in the later study, mean D ratio and mean P ratio among Wolfram patients was compared with the cutoff, to better understand the type of hydrouretronephrosis among this group and to come up with proper treatments based on the type of hydrouretronephrosis.
Statistical analysis was performed using the Statistical Package for the Social Sciences software (SPSS, version 22; Chicago, Illinois, USA). Continuous variables were expressed as mean ± SD and compared using the simple t-test. The Kolmogorov–Smirnov test was used to assess the normality of continuous variables.
4. Results
A total of seven patients, with a mean age of 24.43 ± 4.25 months, previously diagnosed with WS and with hydrouretronephrosis as the indication for DRUS, were identified. This group of patients consisted of 4 females and 3 males. Characteristics of the patients and their imaging details are shown in Table 1.
| Minimum | Maximum | Values | |
|---|---|---|---|
| Age, mo | 19 | 32 | 24.43 ± 4.25 |
| Age at diagnosis, mo | 6 | 18 | 11.43 ± 2.14 |
| Diameter of hydroureter, mm | |||
| Before drainage | 17 | 25 | 20.64 ± 2.73 |
| After drainage | 7 | 16 | 11.07 ± 2.64 |
| Renal parenchymal cortical diameter, mm | |||
| Before drainage | 19 | 33 | 25.36 ± 4.70 |
| After drainage | 7 | 18 | 12.79 ± 3.51 |
aValues are expressed as mean ± SD.
Following the measurements of renal parenchymal diameters and ureteral diameters on longitudinal views before and after a 3-hour drainage, the mentioned equation was used to calculate P ratio and D ratio for each patient. The mean P ratio among patients was 0.50 ± 0.06. The mean D ratio in this group of patients was 0.45 ± 0.13 with a 0.03 standard error of mean. The D ratio was compared to the cutoff point of 0.22 that was earlier introduced in a study done by our center. One-sample t-test was used to examine if any significant difference between the cutoff point and the D ratio in our patients existed, which demonstrated that D ratio was significantly higher in our patients (P value < 0.05). It can be inferred from these calculations that hydrouretronephrosis in patients suffering from WS is probably a non-obstructive type.
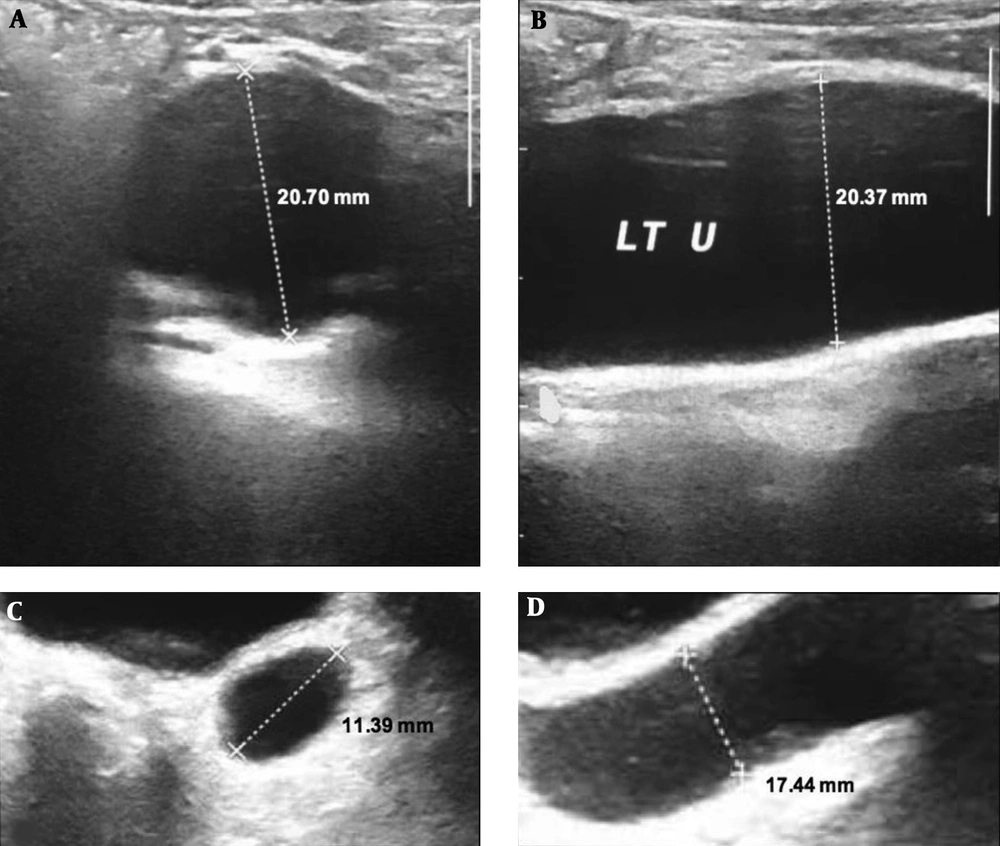
In part A and B patient’s left ureter is displayed prior to free drainage and as labeled, the size of ureter is measured in two views, in part C and D patient’s ureter is demonstrated following two hours of free drainage and as labeled, the ureter size is again measured and there is significant decrease in the caliber of ureter.
5. Discussion
Urological dysfunctions are among common manifestations in WS; and their complication is a common cause of mortality and morbidity in affected patients. Majority of urinary complications are in the form of upper urinary tract dilatation, reported to be present in 90% of patients; however, they remain asymptomatic in many patients. Upper urinary tract dilatation varies from mild hydronephrosis to megaureter (8, 11).
Several theories have been proposed to explain these urologic manifestations. The main hypothesis is that dilated upper tract and large atonic bladder are secondary to other components of the syndrome including polyuria due to diabetes insipidus and diabetes mellitus or autonomic nerve dysfunction. However, there is also a notion that urologic symptoms might be primary components of WS (8).
Since the therapeutic approach to different types of hydroureters is different, it is critical to distinguish the type of hydroureter. Hydroureters’ types include refluxing and non-refluxing ones and within the non-refluxing hydroureters, there are two subtypes including obstructive and non-obstructive or functional types. Primary non-refluxing non-obstructive hydroureter mostly resolves spontaneously whereas refluxing and/or obstructive hydroureter requires intervention to correct the underlying causes (12).
Misdiagnosis of the type of the hydroureter in WS patients and encountering them as obstructive hydroureters has led to ineffective, unnecessary and invasive procedures for instance ureter re-implantation.
Current established imaging modalities to detect obstruction in urinary tract and to differentiate different types of hydroureters are intravenous ureterography (IVU) and diuretic renography. IVU is the mainstay imaging modality to detect obstructive uropathies and it can assess the function and the structure of the kidney in the same time; however, it produces a high rate of false-positive results or indeterminate findings and it also exposes the child to ionizing radiation with long-term consequences (13).
Diuretic renography can potentially differentiate distinct degrees of obstruction and it is beneficial for evaluation of differential function at diagnosis as well as during follow-up of primary megaureter. On the other hand, as it requires longer time and patients’ steadiness during the imaging its use is limited (14, 15).
The use of Fluorescence imaging as an alternative to radiological modality to detect ureteropelvic junction obstruction (UPJO) has been proposed but it is only limited to animal studies and has not been applied to differentiate obstructive from non-obstructive hydroureter (16).
Magnetic resonance urography (MRU) is a highly sensitive means to detect the obstruction, it provides detailed information on morphological features and does not expose the child to radiation but MRU is expensive, requires sedation and lasts up to one and half hours (17).
Computed tomography urography (CTU) is promising to evaluate ureteral obstruction but besides the contrast media reaction, the use of CT scan is very limited in pediatric population because of the high amount of radiation exposure (18).
Other studies explored the use of color Doppler sonography as a modality of discrimination between two types of hydroureter and assessed the relative jet frequency less than 25% as a good indicator of obstruction in severe unilateral hydronephrosis but its ability in determining obstruction at some levels is yet to be defined (19).
In 2015 Kajbafzadeh et al. (10) proposed DRUS as a beneficial means of discriminating obstructive and non-obstructive hydroureters and they also claimed that DRUS can differentiate the level of the obstruction. They developed a formula as D ratio= [(|D1-D2|)/D1] × 100 in which D1 referred to the largest diameter of the ureter, immediately after the insertion of bladder catheter, and D2 presented the same value after 3 h of catheterization. It has been showed that by cutoff value of 22% of D ratio with a sensitivity of 78.5% and specificity of 83.4% we can predict the presence of the obstruction. The free drainage provided by DRUS by increasing the urine flow, decreases the bladder pressure in non-obstructive uropathies and hereby differentiates the underlying etiology of the hydroureter (10). DRUS has the advantage of being cost-effective and no radiation exposure but it also has been known that like any other sonography related modality, DRUS is associated with some degree of operator dependence. Repeated catheterization as the only invasiveness of DRUS has been minimized by performing the VCUG at the end of DRUS and omitting the free bladder drainage phase of diuretic renogram.
In this study we demonstrated the application of DRUS in WS, as a modality to distinguish non-obstructive hydroureters from obstructive ones. By the aid of DRUS, non-obstructive hydroureters can be recognized in patients with WS, and as non-obstructive hydroureters would not benefit from reconstructive surgeries, unnecessary surgeries could be limited by using this diagnostic tool. Furthermore, patients with non-obstructive hydroureters would be good candidates for intermittent catheterization. Also as we have discussed in an earlier study in our center, performing an appendicovesicostomy with the Mitrofanoff principle in patients with WS that would benefit from clean intermittent catheterization, could help patients to independently perform CIC even with progression of visual loss (20). The main limitation of this study is its small sample size, which could be acceptable based on Wolfram syndrome’s rareness.
To sum up, it is essential to consider DRUS as a diagnostic tool for establishing hydroureters’ type in WS, and limiting unnecessary surgeries, that are beneficial only for obstructive hydroureterss.
5.1. Conclusions
Treatment approach to hydroureteronephrosis is dependant on whether it is obstructive or non-obstructive, therefore distinguishing this characteristic is critical. The present study, demonstrated efficacy of DRUS in differentiating obstructive and non-obstructive hydroureters with high sensitivity in patients with WS. It also emphasized on this modality’s accessibility and low-cost. Furthermore, DRUS would limit patients’ exposure to unnecessary radiation and therefore could be accounted as a conventional imaging method in WS patients.