1. Background
Acute lymphoblastic leukemia (ALL) is the most common pediatric malignancy worldwide, comprising 20% to 30% of childhood cancers (1, 2). Children with ALL frequently need use of different blood products during their treatment (3, 4) due to chemotherapy-induced myelosuppression superimposed on an already dysfunctional marrow (5). Accordingly, being affected by both their disease and treatment, leukemia patients are considered unique in terms of their universal requirement for both erythrocyte and platelet transfusions (5).
However, due to lack of standardized evidence-based guidelines for blood product transfusions, a wide variation exists in blood product transfusion practices across several clinical settings (6). Moreover, along with lack of studies that systematically address the transfusion requirements and triggers in acute leukemia (5), no study to date has addressed the amount as well as efficacy of blood product use with respect to type of chemotherapeutics in pediatric ALL patients under chemotherapy (CT).
2. Objectives
The present study was therefore designed to evaluate for the first time in the literature, total erythrocyte, random platelet and apheresis platelet suspension requirements and efficacy of blood transfusion in pediatric ALL patients in relation to different CT protocols.
3. Methods
3.1. Study Population
A total of 172 patients diagnosed with ALL were enrolled in this retrospective study conducted at Ondokuz Mayis University, Faculty of Medicine, Pediatric Hematology clinics between January 2005 and January 2014. Of 172 patients initially enrolled, 146 patients were subjected to the final analysis after exclusion of 26 patients due to diagnosis of bi-phenotypic ALL (n = 3), receiving ALL St Jude protocol (n = 8), follow up at another hospital (n = 5) and missing data on blood bank or hospital information management system (n = 10).
3.2. Assessments
Data on patient demographics (age, gender), age at diagnosis, type of CT protocol, completion of CT protocol and amount of blood product use (erythrocytes, apheresis platelet and random platelet) and complete blood count (CBC) findings 3 days before and 3 days after the transfusion including hemoglobin (Hb), white blood cell (WBC), neutrophil, lymphocyte and erythrocyte counts and red cell distribution width (RDW) were retrieved from hospital records as was the survival outcome during CT.
3.3. CT Protocols
ALL BFM 2002 and ALL IC 2009 treatment protocols (7) are applied in our clinic in routine management of ALL patients. In the standard risk group, treatment schedule starts with protocol 1a and ends with protocol 1b, protocol M, protocol 2 and maintenance. In the moderate risk group treatment schedule starts with protocol 1a and continues with protocol 1b (in T-ALL), or protocol 1b augmented (in B-ALL) as followed by protocol M, protocol 2 and maintenance. In the high risk group, treatment schedule starts with protocol 1a and ends with protocol 1b (in T-ALL), or protocol 1b augmented (in B-ALL) as followed by HR 1a, HR 2a, HR 3a, HR 1b, HR 2b, HR 3b, protocol M, protocol 2 and maintenance.
Protocol 1A includes prednisone/prednisolone (60 mg/m2/day, po/iv, 1st - 28th day), vincristine (1,5 mg/m2/day - max 2 mg, iv, 8th, 15th, 22nd, and 29th day), daunorubicin/doxorubicine (30 mg/m2/day, 8th, 15th day for SR patient, 8th, 15th, 22nd, and 29th day for IR/HR patients), E. coli asparaginase (5000 U/m2/day, iv, 12th, 15th, 18th, 21st, 24th, 27th, 30th, and 3 day).
Protocol 1b includes cyclophosphamide (1000 mg/m2/day, iv; 36th, and 64th day), 6-mercaptopurine (60 mg/m2/day, po, 36th-63rd day), Ara-C (75 mg/m2/day, iv, 38th - 41st, 45th - 48th, 52nd - 55th, and 59th - 62nd day) and protocol 1b augmented cyclophosphamide (1000 mg/m2/day, iv; 36th and 64th day), 6-mercaptopurine (60 mg/m2/day, po, 36th - 49th and 64th - 77th day), Ara-C (75 mg/m2/day, iv, 37th - 40th, 43rd - 46th, 65th - 68th, and 72nd - 75th day), vincristine (1,5 mg/m2/day, iv, 50th, 57th, 78th, and 85th day), E. coli asparaginase (5000 U/m2/day, iv, 50th, 52nd, 54th, 57th, 59th, 61st, 78th, 80th, 82nd, 85th, 87th, and 89th day).
In protocol M, SR patient is given methotrexate 2000 mg/dose, 24 hour infusion, totally 4 doses, doses 14 days apart from each other, starting on day 8, in this protocol HR and IR patients receive (MTX) 5000 mg/dose, as 24 hour infusion, totally 4 doses, 14 days apart from each other starting on day 8. During treatment with Protocol M both risk groups receive oral mercaptopurine (MCP) 25 mg/m2/day for 1 - 56 days.
Protocol 2 includes dexamethasone (10 mg/m2/day, PO, 1 - 21 day), vincristine (1,5 mg/m2/day, IV, 8, 15, 22, and 29 day), doxorubicine (30 mg/m2/day, iv, 8, 15, 22, and 29.day), E. coli asparaginase (10000 U/m2/day, IV, 8, 11, 15, 18 day), cyclophosphamide (1000 mg/m2/day, IV, 36 day), 6-thioguanin (60 mg/m2/day, po, 36 – 49 day), Ara-C (75 mg/m2/day, iv, 38 - 41 and 45 - 48 day).
HR-1 protocol includes dexamethasone (20 mg/m2/day, po, 1 - 5 days), vincristine (1,5 mg/m2/day, iv, on day 1 and 6), Methotrexate (5000 mg/m2/day, 24 hour infusion, on day 1), cyclophosphamide (200 mg/m2/day, iv, on day 2 - 4), cytarabine (2000 mg/m2/dose, 2 doses 12 hours apart, starting on day 5), E. coli asparaginase (25000 U/m2, iv, on day 6).
HR-2 protocol includes dexamethasone (20 mg/m2/day, po, 1 - 5 days), vincristine (1,5 mg/m2/day, iv, day 1 and 6), Methotrexate (5000 mg/m2/day, 24 hour infusion, day 1), ifosfamide (800 mg/m2/dose, 5 doses 12 hours apart, starting on day 2), E. coli asparaginase (25000 U/m2/iv, on day 6), daunorubicin (30 mg/m2/day, iv, on day 5).
HR-3 protocol includes dexamethasone (20 mg/m2/day, po, 1 - 5 days), cyclophosphamide (200 mg/m2/day, iv, 2 - 4 days), cytarabine (2000 mg/m2/dose, 4 doses 12 hours apart, on day 1 and 2), E. coli asparaginase (25000 U/m2, iv, on day 6), etoposide (100 mg/m2/dose, 5 doses 12 hours apart, on day 3 - 5).
HR patients were given 2 times HR-1, HR-2, HR-3 protocol 21 days apart from each other. Further in text first protocol is designated HR-1a for the first and HR-1b for the second treatment with protocol HR-1 while drugs and doses remain the same.
All patients with ALL were put on oral maintenance therapy (MT) with daily 6-MCP (50 mg/m2) and weekly MTX (20 mg/m2). The overall duration of treatment from the start of induction through the end of MT is uniformly 104 weeks.
3.4. Blood Product Use
The total number of erythrocyte, random platelet and apheresis platelet suspension requirements of each ALL patient from the date of diagnosis to the end of the treatment were retrieved from blood bank records. The use of blood products between CT protocols were recorded based on total number of blood cells and bags.
In our department, erythrocyte transfusion indication in children with ALL includes administration of 15 mL/kg (500 mL at most) erythrocyte when blood hemoglobin concentration is lower than 8 g/dL or hematocrit level is lower than 24%. The dose of erythrocyte suspension is 15 mL/kg for patients ≤ 20 kg and 500 mL (two units) at maximum for patients > 20 kg. In the blood bank of our hospital, erythrocyte suspension with an average volume of 250 mL per unit (one bag = one unit) prepared with saline + adenine + glucose + mannitol (SAG-M) with 55% - 60 % hematocrit is used and it is kept up to 42 days at 1°C - 6°C in refrigerator. However, the erythrocytes used for patients with acute leukemia are 5 - 7 days old erythrocytes in the form of erythrocyte suspension irradiated at 2500 cGy dose and they undergo inline leukocyte filter application before being stored in the blood bank.
Platelets were kept at 20°C - 24°C in the blood bank of our hospital and due to risk for bacterial infection at this temperature; the period of keeping platelet is limited to only five days. After five days, platelets lose their vitality by 20% - 25%. In our hospital, there are two types of platelet suspension as apheresis and random with a unit (one bag = one unit) volume of 60 mL on average. Our first choice in patients with thrombocytopenia is administration of apheresis platelets, given that it includes more intense platelets in smaller volume (a unit of apheresis platelet suspension corresponds to 6 - 8 random-donor (random) platelet suspension in terms of the number of platelets). If apheresis platelet cannot be obtained, random platelet suspension is given calculated as one unit for 10 kg. In our hospital, the criteria for platelet suspension treatment in children with ALL is platelet levels of < 20.000/µL under normal conditions and platelet levels of < 30.000/µL in the course of infection.
3.5. Statistical Analysis
Statistical analysis was made using IBM SPSS Statistics for Windows, version 21.0 (IBM Corp., Armonk, NY). Normality assumption was tested with Kolmogorov-Smirnov test. Kruskal Wallis with Bonferroni correction and Mann-Whitney U test were used for analysis of continuous data. Data were expressed as median (minimum-maximum) and percent (%) where appropriate. P < 0.05 was considered statistically significant.
4. Results
4.1. Demographic and Clinical Characteristics and Survivorship Status
Overall, males comprised 54.0% of the study population and 32.0% of patients were in the 1 - 9 years age group. Distribution of CT protocols is summarized in Table 1.
| Variables | Values |
|---|---|
| Age, y | |
| ≤ 1 and ≥ 9 | 100 (68.0) |
| 1 - 9 | 46 (32.0) |
| Gender | |
| Male | 78 (54.0) |
| Female | 68 (46.0) |
| CT protocol | |
| Protocol 1a | 145 (99.0) |
| Protocol 1b | 67 (46.0) |
| Protocol 1b augmented | 77 (52.0) |
| HR 1a | 57 (39.0) |
| HR 2a | 54 (37.0) |
| HR 3a | 53 (36.0) |
| HR 1b | 50 (34.0) |
| HR 2b | 50 (34.0) |
| HR 3b | 49 (33.0) |
| Protocol M | 87 (60.0) |
| Protocol 2 | 134 (91.0) |
| Maintenance | 126 (86.0) |
| Survivorship status | |
| Survivor | 127 (87.0) |
| Non-survivor | 19 (13.0) |
| Male | 8 (10.2) |
| Female | 11 (16.2) |
| Last CT regimen by non-survivors | |
| Protocol 1b | 1 (5.0) |
| HR1a | 1 (5.0) |
| HR3a | 1 (5.0) |
| HR2b | 1 (5.0) |
| Protocol 2 | 6 (32.0) |
| Maintenance | 9 (48.0) |
aValues are expressed as No. (%).
In total 19 (13.0%) patients, 11 females and 8 males, did not survive to complete the treatment. The last CT regimen before death was maintenance in 9 (48.0%) patients and protocol 2 in 6 (32.0%) patients, while Protocol 1, HR 1a, HR 3a and HR 2b were the other final CT regimens among non-survivors, each in 1 (5.0%) patient (Table 1).
4.2. Blood Product Use According to CT Regimens
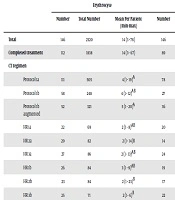
The total number of erythrocytes, apheresis platelets and random platelets received by 146 ALL patients from the date of diagnosis were 2320 [median 14 (range, 3- 78)] bags, 1676 [median 9 (range, 1 - 97)] bags and 1533 [median 11 (range, 1 - 83)] bags, respectively. In those who completed all steps of CT protocols (n = 112), the total number of erythrocytes, apheresis platelets and random platelets were 1838 [median 14 (range, 3 - 67)] bags, 1046 [median 8 (range, 1 - 56)] bags and 1132 [median 10 (range, 1 - 83)] bags, respectively (Table 2). Protocol 1b augmented was associated with the highest amount of erythrocyte use (P < 0.001) followed by HR 2a, HR 2b, protocol M and HR 3b protocols, while erythrocyte use was also significantly higher in protocol 1a and protocol 2 than in HR 2a protocol (P < 0.001) (Table 2).
| Erythrocyte | Apheresis Platelet | Random Platelet | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Number | Total Number | Mean Per Patient (min-max) | Number | Total Number | Mean Per Patient (min-max) | Number | Total Number | Mean Per Patient (min-max) | |
| Total | 146 | 2320 | 14 (3 - 78) | 146 | 1676 | 9 (1 - 97) | 146 | 1533 | 11 (1 - 83) |
| Completed treatment | 112 | 1838 | 14 (3 - 67) | 89 | 1046 | 8 (1 - 56) | 77 | 1132 | 10 (1 - 83) |
| CT regimen | |||||||||
| Protocol 1a | 111 | 505 | 4 (1 - 19)A | 78 | 381 | 3 (1 - 33) | 49 | 405 | 6 (1 - 26) |
| Protocol 1b | 58 | 248 | 4 (1 - 12)AB | 27 | 59 | 2 (1 - 8) | 18 | 107 | 4.5 (2 - 17) |
| Protocol 1b augmented | 52 | 321 | 5 (1 - 20)A | 36 | 141 | 3 (1 - 17) | 23 | 158 | 4 (1 - 43) |
| HR 1a | 22 | 69 | 2 (1 - 8)AB | 20 | 54 | 2 (1 - 15) | 11 | 45 | 4 (1 - 8) |
| HR 2a | 29 | 82 | 2 (1 - 14)B | 14 | 54 | 2 (1 - 14) | 4 | 38 | 7.5 (4 - 19) |
| HR 3a | 27 | 86 | 2 (1 - 13)AB | 24 | 77 | 1 (1 - 25) | 12 | 71 | 5 (2 - 20) |
| HR 1b | 26 | 84 | 3 (1 - 9)AB | 19 | 59 | 1 (1 - 11) | 11 | 61 | 4 (2 - 12) |
| HR 2b | 23 | 84 | 2 (1 - 23)B | 17 | 114 | 2 (1 - 57) | 7 | 27 | 4 (2 - 6) |
| HR 3b | 25 | 71 | 2 (1 - 6)B | 22 | 58 | 2 (1 - 11) | 11 | 61 | 4 (2 - 17) |
| Protocol M | 28 | 89 | 2 (1 - 9)B | 18 | 84 | 2 (1 - 24) | 19 | 119 | 5 (2 - 22) |
| Protocol 2 | 81 | 500 | 4 (1 - 49)A | 61 | 427 | 3 (1 - 71) | 32 | 277 | 5.5 (1 - 44) |
| Maintenance | 37 | 181 | 3 (1 - 19)AB | 29 | 168 | 2 (1 - 32) | 18 | 164 | 6 (2 - 27) |
| P value | < 0.001 | 0.050 | 0.132 | ||||||
aThere are many P values for comparing protocols, so there is a practical way to understand which CT protocol has a significant difference or not. If the variables have same letter like A, B, or AB this means there is no significant difference between the variables. If the variables have different letter like A vs B or A vs AB, this means there is a significant difference between these protocols with P value < 0.05. Single P values represent comparison of columns. The three P values in the last raw compare the related columns.
No significant difference was noted in apheresis platelet and random platelet use with respect to CT regimens (Table 2).
4.3. CBC Findings After Transfusion of Blood Products
Erythrocyte transfusion was associated with a more marked increase in Hb, RBC, WBC, lymphocyte, and neutrophil counts as compared with apheresis platelet and random platelet infusions, while increase in RDW and thrombocyte values was more pronounced in apheresis platelet and random platelet transfusions than in erythrocyte transfusion (P < 0.001 for each) (Table 3).
| Change From Pre-Transfusion Value | |||
|---|---|---|---|
| Erythrocytes | Apheresis Platelets | Random Platelets | |
| Hemoglobin | |||
| Total | 2.7 (-3.22 - 9.8)B | -0.4 (-4.7 - 7.2)A | -0.6 (-4.4 - 5.7)A |
| P value | < 0.001 | ||
| According to CT regimens | |||
| Protocol 1a | 2.7 (-3.22:9.8)AB | -0.2 (-2.67:5.1)AB | -0.5 (-4.4:1.5) |
| Protocol 1b | 2.5 (-0.3:8.4)A | -0.6 (-3:4.5)AB | -0.2 (-2:3.8) |
| Protocol 1 b augmented | 2.7 (-0.7:6.2)AB | -0.32 (-2.2:7.2)AB | -0.5 (-2.3:1.9) |
| HR 1a | 2.7 (0.4:5.5)AB | -0.3 (-2.3:3.2)AB | -1 (-1.9:2.7) |
| HR 2a | 2.69 (1.1:4.6)AB | -0.4 (-1.9:2.7)AB | -1 (-1.8:-0.25) |
| HR 3a | 2.49 (0:4.65)AB | -1.46 (-4.7:6.17)A | -1.1 (-1.44:2.4) |
| HR 1b | 2.7 (-1:5.5) AB | -0.3 (-2.8:4.1)AB | 0 (-1.5:2.3) |
| HR 2b | 2.3 (0.4:5.4)AB | -0.3 (-4.2:4.4)AB | -0.45 (-1:0.1) |
| HR 3 b | 2.9 (0.4:7.3)AB | -0.4 (-3.6:2.6) AB | -1.15 (-1.6:1.01) |
| Protocol M | 2.9 (0:7.3)AB | -0.2 (-2.6:5.7)B | -0.8 (-3.6:5.7) |
| Protocol 2 | 3.2 (-0.7:7.14)B | -0.6 (-2.6:3.2)AB | -0.8 (-2.7:4.2) |
| Maintenance | 2.9 (-0.2:7.5)AB | -0.2 (-2.4:5.2)AB | -0.7 (-2.1:1.9) |
| P value | 0.017 | 0.004 | 0.825 |
| RBC | |||
| Total | 0.9(-1.05 - 3.97)B | -0.12 (-1.66 - 2.48)A | -0.2 (-1.49 - 1.91)A |
| P value | < 0.001 | ||
| According to CT regimens | |||
| Protocol 1a | 0.88 (-1.05:3.97)AB | -0.08 (-1.17:1.67)AB | -0.16 (-1.49:0.53) |
| Protocol 1b | 0.87 (-0.18:2.79)A | -0.16 (-0.98:1.74)AB | 0.01 (-0.71:1.36) |
| Protocol 1 b augmented | 0.87 (-0.36:2.02)AB | -0.13 (-0.78:2.48)AB | -0.15 (-0.81:0.7) |
| HR 1a | 0.85 (0.09:1.7)AB | -0.05 (-0.74:1.04)AB | -0.3 (-0.64:0.94) |
| HR 2a | 0.84 (0.32:1.47)AB | -0.14 (-0.64:0.85)AB | -0.27 (-0.64:-0.12) |
| HR 3a | 0.82 (0.2:1.63)AB | -0.4 (-0.82:1.84)A | -0.32 (-0.54:0.74) |
| HR 1b | 0.89 (-0.38:1.94)AB | -0.13 (-0.93:1.03)AB | -0.03 (-0.56:0.77) |
| HR 2b | 0.69 (0:1.76)AB | -0.07 (-1.66:1.38)AB | -0.08 (-0.17:0.02) |
| HR 3b | 0.9 (0.21:2.5)AB | -0.12 (-1.19:0.81)AB | -0.33 (-0.51:0.19) |
| Protocol M | 0.9 (-0.13:2.42)AB | 0.04 (-0.94:1.91)B | -0.3 (-1.21:1.91) |
| Protocol 2 | 1.08 (-0.24:2.2)B | -0.17 (-1.12:1.04)A | -0.24 (-0.87:1.25) |
| Maintenance | 0.97 (-0.33:2.67)AB | 0.03 (-0.87:1.64)AB | -0.12 (-0.52:0.74) |
| P value | 0.027 | 0.011 | 0.648 |
| RDW | |||
| Total | -0.4(-7 - 22.6)B | -0.1(-18 - 2.8)A | 0(-2.5 - 2.5)A |
| P value | < 0.001 | ||
| According to CT regimens | |||
| Protocol 1a | -0.6 (-6.9:22.6) | -0.1 (-18:2.8) | 0 (-1.3:1.6) |
| Protocol 1b | -0.5 (-6:11) | -0.1 (-1.9:1.4) | 0.3 (-2.4:2.3) |
| Protocol 1 b augmented | -0.4 (-5.1:3.5) | 0 (-2.4:2.4) | 0 (-0.9:2.3) |
| HR 1a | -0.2 (-2.6:1.1) | -0.1 (-1.2:0.6) | -0.15 (-0.4:1) |
| HR 2a | -0.6 (-2.4:2) | -0.1 (-0.9:1.4) | -0.15 (-1:0.3) |
| HR 3a | 0.1 (-3.7:4.6) | -0.1 (-2.7:2.5) | 0.45 (-0.5:2.5) |
| HR 1b | -0.3 (-3.5:3.7) | 0 (-0.9:2.1) | -0.5 (-1.9:0.1) |
| HR 2b | -0.45 (-3.4:3.3) | 0 (-3.4:1.9) | -0.1 (-0.3:0.1) |
| HR 3b | -1.1 (-3.3:0.8) | -0.15 (-2.1:2.1) | -0.45 (-2.4:0.4) |
| Protocol M | -0.5 (-4.1:2.3) | -0.1 (-3.1:2.1) | 0 (-0.8:1.3) |
| Protocol 2 | -0.3 (-7:6.1) | -0.1 (-7:2.6) | 0 (-1.8:1.8) |
| Maintenance | -0.4 (-4.1:8.2) | 0.05 (-1.6:2.4) | -0.2 (-2.5:2.5) |
| P value | 0.116 | 0.920 | 0.186 |
| WBC | |||
| Total | 0.11(-43.5 - 55.5)B | 0(-162.8 - 76.7)A | -0.09 (-62.76 - 8.7)A |
| P value | < 0.001 | ||
| According to CT regimens | |||
| Protocol 1a | 0.04 (-43.5:55.5) | -0.07 (-162.8:30) | -0.55 (-62.76:3.34) |
| Protocol 1b | 0.2 (-2.2:9.3) | 0.1 (-1.4:3) | 0.1 (-0.8:1.5) |
| Protocol 1 b augmented | 0.1 (-3.76:6.4) | -0.1 (-6.1:13.25) | -0.1 (-7:8.7) |
| HR 1a | 0.15 (-1.62:2.39) | 0 (-1.43:76.7) | 0.09 (-0.21:2.74) |
| HR 2a | 0.3 (-1.73:6.3) | 0.04 (-1.8:4.9) | -0.91 (-30:0.37) |
| HR 3a | -0.02 (-4.96:1.3) | 0.01 (-14.57:10.79) | 0.08 (-23.9:0.8) |
| HR 1b | 0.44 (-6.93:9.1) | -0.04 (-1.96:1.8) | -0.08 (-3.02:1.42) |
| HR 2b | 0.18 (-1.93:12.98) | -0.02 (-13.22:14.8) | 1.11 (0:2.21) |
| HR 3b | 0.12 (-1.41:9.5) | 0.05 (-9.48:5.15) | -0.16 (-19.4:0.6) |
| Protocol M | 0.06 (-5.3:2.38) | 0.03 (-1.15:3.34) | -0.12 (-6.5:8.2) |
| Protocol 2 | 0.1 (-25.6:4.71) | 0 (-10.86:3.7) | -0.03 (-3.7:2.44) |
| Maintenance | 0.2 (-3.57:5.35) | 0.05 (-119:7.5) | -0.35 (-1.49:2.63) |
| P value | 0.050 | 0.164 | 0.177 |
| Lymphocytes | |||
| Total | 0.02(-90-26.4)B | 0(-106-107.3)A | 0(-35.05-13.9)A |
| P value | 0.001 | ||
| According to CT regimens | |||
| Protocol 1a | 0 (-90:26.4) | -0.12 (-106:51.88) | -0.35 (-35.05:3.16) |
| Protocol 1b | 0.03 (-2.2:6.15) | 0.02 (-0.6:1) | 0.1 (-0.7:1.15) |
| Protocol 1 b augmented | 0 (-2.4:6.8) | 0 (-1.05:1.48) | 0 (-5.5:13.9) |
| HR 1a | 0.1 (-1.39:1.43) | 0.01 (-1.1:107.3) | 0.04 (-0.17:1.28) |
| HR 2a | 0.1 (-2.6:1.19) | 0.03 (-0.8:0.6) | -0.1 (-17.06:0.97) |
| HR 3a | 0.01 (-3.18:0.77) | -0.01 (-17.05:0.88) | 0.05 (-19.5:0.3) |
| HR 1b | 0.12 (-0.57:0.8) | -0.01 (-1.81:0.8) | -0.13 (-0.63:0.67) |
| HR 2 b | 0.01 (-1.79:2.29) | 0 (-12.43:13.1) | 0.4 (0.1:0.7) |
| HR 3 b | 0.06 (-0.3:5.3) | 0.01 (-5.56:7.9) | -0.15 (-14.81:0.01) |
| Protocol M | 0.02 (-0.8:0.68) | 0.01 (-0.95:0.8) | -0.06 (-1.1:0.7) |
| Protocol 2 | 0.02 (-16.7:1.2) | 0.01 (-3.33:3) | 0.04 (-4:1.57) |
| Maintenance | 0.1 (-1.52:0.9) | 0.02 (-91.7:1.3) | 0 (-1.57:1.1) |
| P value | 0.135 | 0.050 | 0.198 |
| Neutrophils | |||
| Total | 0(-7.1 - 55.1)B | 0(-200.67 - 9.65)A | 0(-12.9 - 8.6)A |
| P value | < 0.001 | ||
| According to CT regimens | |||
| Protocol 1a | 0 (-7.1:55.1) | 0 (-200.67:8.6) | 0 (-8.01:0.8) |
| Protocol 1b | 0 (-1.23:8.34) | 0 (-1.15:1.4) | 0 (-0.4:0.1) |
| Protocol 1b augmented | 0.03 (-4.08:5.68) | 0 (-6.09:9.65) | -0.02 (-1.4:0.22) |
| HR 1a | 0 (-1.35:2.51) | -0.01 (-28.6:2.92) | 0.02 (-0.06:0.17) |
| HR 2a | 0.1 (-1.61:6.5) | 0.04 (-2.3:5.45) | -1.12 (-12.9:-0.06) |
| HR 3a | 0 (-5.62:0.86) | 0 (-0.9:8.3) | 0 (-0.03:0.02) |
| HR 1b | 0.04 (-6.21:6.4) | -0.01 (-0.3:1.14) | 0.02 (-2.31:0.25) |
| HR 2b | 0.01 (-1.89:10.96) | -0.03 (-1.46:0.9) | 0.05 (-0.09:0.18) |
| HR 3b | 0 (-1.52:2.93) | 0 (-26.01:4.98) | -0.03 (-0.6:0.1) |
| Protocol M | 0.02 (-4.7:2.23) | 0.01 (-0.37:2.6) | -0.04 (-6.1:8.6) |
| Protocol 2 | 0.02 (-6.24:4.66) | 0 (-6.54:2.48) | 0 (-2.03:1.61) |
| Maintenance | 0 (-3.87:4.72) | 0 (-12.8:7.3) | -0.1 (-1.13:2.04) |
| P value | 0.425 | 0.313 | 0.077 |
| Thrombocytes | |||
| Total | -8.8 (-659-484)B | 28 (-173-460)A | 15 (-411-247)A |
| P value | < 0.001 | ||
| According to CT regimens | |||
| Protocol 1a | -5.8 (-659:155) | 18.15 (-95:408)A | 17.5 (-411:247) |
| Protocol 1b | -18 (-233:484) | 66 (-39:187)B | 15 (-4:57) |
| Protocol 1 b augmented | -15 (-163:133) | 36 (-76:256)AB | 12.91 (-10.5:86) |
| HR 1a | -3 (-58:185) | 34 (-27:191)AB | 18 (0:191) |
| HR 2a | -9 (-189:123) | 22 (-48:155)AB | 10.6 (-13:16) |
| HR 3a | -0.4 (-111:200) | 36 (-19.08:200)AB | 10.5 (-1.8:35) |
| HR 1b | 3 (-221:254) | 32 (-35:208)AB | 19 (3.4:50) |
| HR 2b | 1.5 (-55:151) | 25 (-47:152)AB | 55 (20:90) |
| HR 3b | -9.8 (-96:22) | 23.5 (-20:460)AB | 21.5 (14.5:154) |
| Protocol M | -8 (-106:214) | 33 (-25:207)AB | 18 (-24:88) |
| Protocol 2 | -7 (-143:191) | 21 (-173:172)A | 7.73 (-13:178) |
| Maintenance | -4 (-108:294) | 32 (-34:382)AB | 14 (-22:104) |
| P value | 0.050 | 0.006 | 0.153 |
aValues are expressed as median (min:max) or meadian (range).
bThere are many P values for comparing protocols, so there is a practical way to understand which CT protocol has a significance difference or not. If the variables have same letter like A, B, or AB this means there is no significant difference between the variables. If the variables have different letter like A vs B or A vs AB, this means there is a significant difference between these protocols with P value < 0.05. Single P values represent comparison of columns. The three P values in the last raw compare the related columns.
When the impact of blood transfusion on CBC findings was evaluated according to CT regimen in each transfusion group, protocol 2 vs protocol 1b was associated with achievement of higher Hb (P = 0.017) and RBC (P = 0.027) levels after erythrocyte transfusion. Protocol M was associated with achievement of higher Hb (vs HR 3a, P = 0.004) and RBC levels (vs both HR 3a and protocol 2, P = 0.011) after apheresis transfusion. In addition, protocol 1b was associated with higher thrombocyte count after apheresis transfusion compared with protocol 1a and protocol 2 (P = 0.006) (Table 3).
5. Discussion
The patient profile (54.0% males) and survival outcome (87% survival rate) in the present cohort of ALL patients seem to be consistent with higher prevalence of disease among males (8) as well as improvements in survival of children with ALL due to earlier diagnosis and advances in therapy (9, 10). However, boys (89.7%) and girls (83.8%) had similarly favorable survival outcome in our cohort, in contrast to a poorer prognosis reported in boys than in girls for the ALL patients under the same CT in the past studies (9, 11).
Our findings revealed the high need for blood products during chemotherapy of ALL patients with an average of 14, 9, and 11 bags of erythrocytes, apheresis platelets and random platelets received during the treatment course. This supports the frequent need for blood transfusions during therapy by most children with ALL, particularly during the induction period (3, 4).
Anemia is considered as a frequent finding not only in the most common manifestations of ALL but also an adverse effect of CT for ALL due to myelosuppression in induction and consolidation phases of CT (12, 13). Accordingly, RBC transfusion is considered critical in ALL patients for reduction of the potential complications of severe anemia (13) with 30 to 60 units of RBCs required to support a leukemia patient through induction therapy (5, 14).
Notably, our findings revealed considerable change in erythrocyte use, but not in apheresis or random platelet use, with respect to CT regimens among ALL patients. In a past study among children with ALL, the number of RBC transfusions and platelet transfusions was reported to be associated with the treatment protocol (15). In our cohort, protocol 1b augmented was associated with the highest amount of erythrocyte use. This seems to be related to association of protocol 1b augmented with a marked bone marrow suppression since it is a prolonged (from 36th to 92nd days) protocol consisted of intense chemotherapeutics such as L-asparaginase, cyclophosphamide, Ara-c and vincristine. Hence, our findings seem to indicate a considerable change in erythrocyte requirement according to chemotherapeutics in ALL patients along with the likelihood of an increase in erythrocyte transfusion need in case of a longer duration CT protocol including intensive chemotherapeutics.
Given that no significant difference in erythrocyte transfusion requirement between HR protocols (1a - 1b, 2a - 2b, and 3a - 3b) exists, our findings may also indicate no significant change in erythrocyte transfusion need when similar chemotherapeutics are administered in different time periods. Nonetheless, a longer time interval between HR 3a and HR 3b protocols than the interval between other HR protocols (1a and 1b and 2a and 2b) seems also to be associated with a slight increase noted in erythrocyte need of our patients treated with HR 3a vs HR 3b protocols.
Erythrocyte transfusion was associated with a more marked increase in Hb levels and RBC, WBC, lymphocyte and neutrophil counts as compared with apheresis platelet and random platelet infusions in our cohort. Moreover, implementation of protocol 2 was associated with achievement of higher Hb (average 3.2 g/dL) levels after erythrocyte transfusion in our patients, indicating likelihood of CT protocol to have an impact on efficacy of erythrocyte transfusion in ALL patients. The association of anemia at baseline prior to initiation of chemotherapy with an increased incidence of chemotherapy-induced anemia and higher likelihood of receiving a RBC transfusion is notable as well as correlation of hemoglobin levels with the improved quality of life in cancer patients (16, 17).
Although transfusion requirements and triggers have not been systematically studied in acute leukemia, any unnecessary increase in morbidity or mortality possibly with use of higher transfusion thresholds is not acceptable (5). In this regard, current practice in our center utilizes a Hb transfusion trigger of 8 g/dL, consistent with suggestion of a lower Hb transfusion threshold (7 - 8 g/dL) rather than a higher Hb transfusion threshold (9 - 10 g/dL) based on its association with decrease in the amount of transfusion requirement as well as in mortality and infection rates in a variety of clinical settings (5, 18, 19).
Nonetheless, given the lack of standardized evidence-based guidelines for blood product transfusions, a need for developing specific transfusion goal for blood products for acute leukemia patients has been emphasized for limiting unnecessary transfusions without compromising outcomes (6).
More pronounced increase noted in thrombocyte counts after apheresis platelet and random platelet transfusions than after erythrocyte transfusion in our patients is an expected finding as the dilutional effect of erythrocyte transfusion and higher amount of thrombocyte content in apheresis and random platelet suspensions. In our clinic, ALL patients receive prophylactic thrombocyte infusion with consideration of thrombocyte threshold value of 30/UL - 40.000/UL. Apheresis platelet infusion was associated with higher increase in platelet counts (average 28.000/µL) than random platelet infusion (average 15.000/µL). This is considered for moderate-to-high dose but not low-dose prophylactic thrombocyte infusion to achieve thrombocyte levels of 60.000/mm3 - 80.000/mm3 in patients under CT (20). Notably, protocol 1B was associated with higher thrombocyte count after apheresis transfusion as compared with protocol 1a and protocol 2 in our cohort, emphasizing the likelihood of CT protocol to influence efficacy of platelet transfusion in ALL patients.
The current study, providing data for the first time in the literature on amount and efficacy of blood product use in relation to chemotherapy protocols among pediatric ALL patients, emphasizes frequent transfusion need in ALL patients and informs both families and blood bank authorities about how much the need for transfusion can increase numerically in some steps of the treatment. Our findings revealed that the need in patients using augmented BFM protocol 1b was highest. Hence, albeit the need of blood and blood product transfusions vary within patients, the anticipated median need for blood products at diagnosis and at various blocks of treatment may be helpful for the blood banks, doctors of the respective pediatric hematology-oncology centers to plan as patients are treated.
Retrospective single center design of the present study seems to be the major limitation, preventing to establish the temporality between cause and effect as well as generalization of our findings to overall ALL population. Another limitation of our study was that the need of patients could not be standardized because of infection or hemorrhage status. We excluded patients who underwent stem cell transplantation.
5.1. Conclusions
Our findings in a retrospective cohort of pediatric ALL patients indicate a great amount of blood product transfusion to be required in the children under chemotherapy. Our findings emphasize a considerable change in erythrocyte requirement according to type of chemotherapeutics in ALL patients along with the likelihood of an increase in erythrocyte transfusion need in case of a longer duration of CT protocol with intensive chemotherapeutics. In addition, our findings seem to indicate likelihood of CT protocol to have an impact on efficacy of erythrocyte and platelet transfusion in ALL patients. There is a need for large prospective randomized trials to address requirement of blood products in ALL populations with respect to patient profile, concomitant disorders and treatments, to improve blood product transfusion practice in ALL patients and to prevent unnecessary transfusions.