1. Background
Urinary tract infection (UTI) is a common bacterial disease in young infants. Due to its non-specific symptoms and signs, it is not easy to detect urinary tract infection timely (1). If left untreated, the disease will progress and ascend to renal parenchyma, ending up with an acute pyelonephritis (APN). APN is a much more severe bacterial infection with the consequence of permanent kidney damage in infants (2, 3). Besides, APN needs a longer antibiotic therapy than lower urinary tract infection does (4). Currently, two image strategies, renal ultrasonography and dimercaptosuccinic acid (DMSA) scintigraphy, are utilized to diagnose APN. DMSA scan particularly is the gold standard tool for the evaluation of renal parenchymal defects and permanent renal scarring (3, 5). Nonetheless, due to risks of sedation and radiation exposure, some parents hesitate to accept this modality, not to mention taking in account the feasibility of this expensive equipment in some regional hospitals (6-8). On top of image modalities, disease history and laboratory parameters can also provide some clues of APN diagnosis. So far, several studies have explored the predictors of APN from an initial febrile UTI, which include fever duration, systemic inflammatory markers, and renal structural problems (9-11). Among these inflammatory markers, white blood cell (WBC) count, neutrophil/lymphocyte ratio (NLR), C-reactive protein (CRP), and procalcitonin (PCT) were all correlated with APN (6, 7, 9, 11-13). However, few studies have focused specifically on young infants. In this unique age group, some factors are influenced by perinatal and prenatal insults, and immature innate immune would interfere the response to the inflammation (14).
2. Objectives
The aim of our research was to determine the relationship between APN and the above-mentioned parameters in infants aged less than 4 months.
3. Methods
This retrospective study included patients aged less than 4 months with first time febrile UTI between January 2012 and December 2018 at the Kaohsiung Veterans General Hospital, a tertiary referral center in southern Taiwan, and this study has been approved by the institutional review board in the medical center. Taiwan Society of Neonatology regulates that neonatologist should take the responsibility for the age group less than 4 months. The diagnosis of febrile UTI was based on the following criteria: (1) fever ≥ 38ºC and (2) positive urine culture results (defined as a single organism ≥ 100,000 colony-forming units/mL for midstream clean-catch collection or ≥ 10,000 colony-forming units/mL for catheterization) (15). We prescribed cefazolin and gentamycin as first line empirical antibiotics. These empirical antibiotics were recommended by a previous study in Taiwan (16). The antibiotic was shifted according to the minimum inhibitory concentration (MIC) of urine culture. Patients were divided into two groups according to the presence of renal defects detected on DMSA scans. During the DMSA exam, the coronal, transverse, and sagittal views of each kidney were scanned. The diagnosis of APN was based on focal decreased uptake in at least two views (17). The diagnosis of vesicoureteral reflux (VUR) was based on voiding cystourethrogram (VCUG) or direct radionuclide cystography (DRC) examination. If patients who received DRC had positive finding, we arranged VCUG for VUR grading.
3.1. Data Analysis
Patients’ detailed personal medical history, including age, sex, and fever duration, was recorded. Because the patients’ family was not always able to recall the definite duration of fever, the therapeutic delay time (TDT) was defined as the day before the beginning of antibiotics use. By contrast, the therapeutic response time (TRT) was extracted from medical chart. Therefore, it was defined as the hour between the beginning of antibiotics use and defervescence. Before antibiotics treatment, blood samples were analyzed to provide data on laboratory parameters, namely CBC, neutrophils, lymphocytes, and CRP. Urine samples were collected through midstream clean-catch or catheterization based on the attending physician’s decision. After admission for febrile UTI, renal ultrasonography and DMSA scans were almost always performed within the first 3 days.
3.2. Statistical Methods
Continuous variables were compared using the independent t test, and categorical variables were analyzed using the chi-square test. Continuous data were presented as mean and standard deviation (mean ± SD), and categorical variables were expressed as percentages. Independent risk factors for APN were analyzed using a multiple logistic regression model, and P < 0.05 was considered statistically significant. Receiver operating characteristic (ROC) curves were evaluated to determine the discriminative ability of each parameter for APN. The cut-off values were selected based on the optimal combination of the highest sensitivity and specificity. Statistical analyses were performed using SPSS 20.0 (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, version 20.0. Armonk, NY: IBM corp.).
4. Results
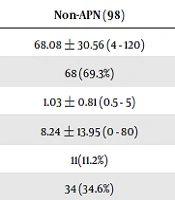
In total, 229 patients were screened, and 19 patients without DMSA exam and 5 patients with congenital renal malformation (2 patients with renal duplication, 2 patients with ureteropelvic junction obstruction; 1 patient with multicystic dysplastic kidney disease) were excluded. According to the DMSA result of 205 patients (age from 4-120 days), 107 patients were included in the APN group, and 98 patients were included in the non-APN group. The clinical characteristics and laboratory findings for the APN group were compared with those for the non-APN group; the results were presented in Table 1. The mean age was 68.73 ± 29.87 days for the APN group and 68.08±30.56 days for the non-APN group (P = 0.878). Both groups predominantly included male patients, but no significant difference was noted (P = 0.581). The TDT, resistance to initial antibiotics, method of urine collection, and urine culture pathogen did not differ significantly between the two groups. In the APN group, 90 patients (84.1%) accepted VUR survey and 31 patients (34.4%) had diagnosed with VUR, and 29 patients of them had grade 3 - 5 VUR. In the non-APN group, 30 patients (30.6%) accepted VUR survey and only 4 patients (13.3%) were diagnosed with VUR, and 2 of them had grade 3 VUR. The incidence rate of VUR is higher in APN group (P = 0.028). Compared with the non-APN group, the APN group exhibited higher TRT (16.99 ± 17.00 h vs. 8.24 ± 13.95 h, P ≤ 0.001), CRP (mg/dL) (6.55 ± 4.16 vs. 3.23 ± 2.87, P < 0.001), and blood NLR (1.89 ± 1.14 vs. 1.31 ± 0.92, P ≤ 0.001). However, the WBC and bacteremia rates difference between the two groups was not statistically significant (Table 1). Multiple logistic regression analysis revealed that CRP, NLR, and TRT were independent risk factors for APN (P ≤ 0.001, 0.003, and 0.004, respectively) (Table 2). The area under the ROC curve (AUC) was 0.774 for CRP (P < 0.001). The optimum cut-off value for CRP was 4.24 mg/dL, which had the highest sensitivity and specificity (70.1% and 73.5%, respectively). AUC was only 0.668 for blood NLR; therefore, this parameter did not exhibit sufficient discrimination for APN prediction (Table 3). Renal ultrasonography finding is organized in Table 4. Only the involved kidney size of APN group and abnormality of reduced cortical vascularity had significant difference.
| Non-APN (98) | APN (107) | P Value | |
|---|---|---|---|
| Age (day) | 68.08 ± 30.56 (4 - 120) | 68.73 ± 29.87 (6 - 118) | 0.878 |
| Male | 68 (69.3%) | 78 (72.8%) | 0.581 |
| Therapeutic delay time (days) | 1.03 ± 0.81 (0.5 - 5) | 1.15 ± 0.82 (0.5 - 3) | 0.284 |
| Therapeutic response time (h) | 8.24 ± 13.95 (0 - 80) | 16.99 ± 17.00 (0 - 81) | < 0.001 |
| Catheterization | 11(11.2%) | 11(10.3%) | 0.828 |
| Resistanceb | 34 (34.6%) | 38 (35.5%) | 0.903 |
| WBC (cells/mm3) | 15162 ± 5590 (5140 - 37440) | 16038 ± 5626 (5400 - 33400) | 0.265 |
| NLR | 1.31 ± 0.92 (0.05 - 5.69) | 1.89 ± 1.14 (0.15 - 5.33) | < 0.001 |
| CRP (mg/dL) | 3.23 ± 2.87 (0.20 - 11.22) | 6.55 ± 4.16 (0.95 - 20.06) | < 0.001 |
| VUR | 4/30 (13.3%) | 31/90 (34.4%) | 0.028 |
| Bacteremia | 2 (2.0%) | 5 (4.6%) | 0.431 |
| Escherichia coli bacteriuria | 92 (93.8%) | 103 (96.2%) | 0.302 |
Abbreviations: APN, acute pyelonephritis; CRP, C-reactive protein; NLR, neutrophil/lymphocyte ratio; VUR, vesicoureteral reflux; WBC, white blood cell
aValues are presented as mean ± standard deviation or percentage (%)
bResistance to initial antibiotics according to the minimum inhibitory concentration of urine culture.
| P Value | Odd Ratio | 95% CI | ||
|---|---|---|---|---|
| Lower | Upper | |||
| WBC | 0.375 | 1.000 | 1.000 | 1.000 |
| NLR | 0.003 | 1.675 | 1.185 | 2.368 |
| CRP | < 0.001 | 1.340 | 1.191 | 1.507 |
| TRT (h) | 0.004 | 1.034 | 1.011 | 1.057 |
Abbreviations: CI, confidence interval; CRP, C-reactive protein; NLR, neutrophil/lymphocyte ratio; TRT, therapeutic response time; WBC, white blood cell
| AUC | Std. Error | P value | 95% CI | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| WBC | 0.547 | 0.040 | 0.240 | 0.469 | 0.626 |
| NLR | 0.668 | 0.038 | < 0.001 | 0.594 | 0.742 |
| CRP | 0.774 | 0.032 | < 0.001 | 0.711 | 0.838 |
Abbreviations: AUC, area under the curve; CI, confidence interval; CRP, C-reactive protein; NLR, neutrophil/lymphocyte ratio; WBC, white blood cell
| Non-APN (98) | APN (107) | P Value | |
|---|---|---|---|
| Involved kidney size (cm) | 5.30 ± 0.45 (4.0 - 6.3)b | 5.59 ± 0.57 (4.2 - 7.2) | < 0.001 |
| Non-involved kidney size (cm) | 5.38 ± 0.49 (4.2 - 6.8) | 0.215 | |
| Hydronephrosis | 4 (4.1%) | 10 (9.3%) | 0.137 |
| Reduced cortical vascularity | 4 (4.1%) | 25 (23.4%) | < 0.001 |
Abbreviation: APN, acute pyelonephritis
aValues are presented as mean ± standard deviation or percentage (%).
bThe mean length of bilateral kidneys in the non-APN group.
5. Discussion
UTI is a common bacterial infection in infants. It causes irreversible renal damage when it progresses to APN. Timely medication administration and adequate antibiotic duration can considerably improve the disease outcome (2, 3). In our study, patients in APN group had higher incidence rate of VUR. Wu et al reviewed 597 infants younger than 1 year old and revealed that a positive DMSA renal scan result was a good indicator for prediction of the possibility of VUR in UTI patients (16). It is important to recognize among UTI patients those who have APN. Both renal ultrasonography and DMSA scans are useful tools to diagnose APN. Com-pared with renal ultrasonography, DMSA scan is a more sensitive method for assessing renal defects (18). However, these two examinations cannot be performed immediately, even at medical centers. Moreover, the sedation and radiation risks to young infants should be considered during the DMSA scan. Although our patients who received DMSA had no obvious side effects immediately, young individuals who have higher sensitivity of growing tissues than adults may have a higher risk for long-term effects from ionizing radiation (19). Because of that, several studies have tried to determine the relationship between APN and inflammation markers. From these studies, WBC count, CRP, erythrocyte sedimentation rate (ESR), PCT, NLR, and fever duration have all been ever proposed as a linkage to APN (6, 7, 9, 11-13, 20-22).
DMSA scans are the gold standard tool for evaluation of renal parenchymal defects and permanent renal scarring (3, 5). With DMSA alone it is difficult to differentiate renal scar from APN and renal scar might be the sequel of previous APN, instead of ongoing inflammatory process. So, our inclusion criteria enrolled first time febrile UTI infants to reduce the possibility of renal scar. Renal hypodysplasia is characterized by reduced kidney size and defective kidney formation. Between the ages of 1 ~ 3 months, the suggested limits of normal kidney size was 3.5 cm ~ 6.5 cm (23). Table 4 shows all renal sizes of the enrolled patients which were all within normal limits in the first time renal ultrasonography. Therefore, the diagnosis of renal hypodysplasia had the minor possibility at that time.
Several researchers have suggested a relationship between APN and CRP. Han et al. demonstrated that CRP was an independent predictor of positive DMSA results (P < 0.001), and that it had acceptable discrimination (AUC: 0.726, P < 0.001) to predict APN in the age group less than 36 months (6). Shaikh et al. used a low CRP value (< 2 mg/dL) to rule out APN. It reduced the probability of APN diagnosis to less than 20% (7). Huang et al. also indicated that CRP had the highest sensitivity and specificity for APN prediction in the mean age group of 16 months. If the CRP level was > 6.64 mg/dL with fever for > 2 days or > 2.73 mg/dL with fever for ≤ 2 days, patients with febrile UTI should be treated under the assumption that they have APN (8). Zhang et al. reviewed 21 studies focusing on age groups less than 16 years and illustrated that CRP had moderate accuracy to diagnose APN resulting from UTI (sensitivity: 82.6%, specificity: 66.9%, AUC: 0.81) (12). Soylu et al. disclosed fever ≥ 38ºC and CRP > 5 mg/dL might be the predictors of VUR and high-grade VUR (24). In our study, the CRP level was higher in the APN group, and it was also an independent risk factor predicting APN. Compared with NLR, only CRP had acceptable discrimination for APN pre-diction in the age group less than 4 months (AUC: 0.774, P < 0.001).
NLR is associated with a systemic bacterial infection in several studies (25-27). In various severe infections, the initial WBC count indicates an elevated neutrophil count and a decreased lymphocyte count (27). Han et al revealed that NLR was higher in the APN group than in the group with simple UTI (P < 0.001) among the age group less than 36 months (6). Other report also indicated higher blood neutrophil percentage and higher NLR are associated with high grade VUR (P < 0.001) (28). In our study, NLR was predominant in the APN group (P ≤ 0.001) and was also an independent predictor for APN diagnosis (P = 0.003). However, NLR did not exhibit sufficient discrimination for APN prediction (AUC: 0.668, P ≤ 0.001).
In contrast to the non-APN group, prolonged fever with longer TDT and TRT were observed in the APN group (8). Gilani et al. demonstrated the relevance of the fever pattern and abnormal DMSA scans for a median age group of 32.6 ± 30.8 months. Both TDT ≥ 48 h and TRT ≥ 24 h predicted an abnormal DMSA scan for the first episode of UTI. If patients had longer TDT and TRT, they were likely to exhibit positive DMSA results (9). The same conclusion for TDT was reached by Fernandez‐Menendez et al. (11). In our study, only TRT was an independent risk factor for APN (16.99 ± 17.00 h vs. 8.24 ± 13.95 h, P ≤ 0.001). TDT was not a significant predictor (1.15 ± 0.82 days vs. 1.03 ± 0.81 days, P = 0.284). In Taiwan, medical treatment is convenient and prompt owing to the compulsory National Health Insurance system. Few infants experienced fever for more than 2 days without receiving medical assistance, which was why TDT in the two groups was similar.
The WBC or leukocyte count can be predictors of APN in older children; they are also related with high-grade APN (13). Ansari Gilani et al. found that a leukocyte count of more than 13,500/mm3 predicted positive DMSA results in the mean age group of 32.6 ± 30.8 months (9). However, in the young infantile period, the WBC range varies widely according to the age. For young infants during the period from birth to 3 months, a WBC count range of 5,000 - 21,000/mm3 is normal (29, 30). The WBC count and differential WBC might be influenced by various factors, including the gestational age, birth weight, and postconceptional age. It is difficult to predict neonatal sepsis other than through the WBC count and differential WBC (26). Manroe et al. also described that if abnormal immature or total neutrophil count was detected, both infectious and noninfectious perinatal insults should be considered in neonates (27). These results explained why the WBC count could not be used to predict APN resulting from febrile UTI in young infants.
PCT and ESR are also related with APN in several studies (6, 10, 20). Leroy et al. reviewed 18 studies including infants with a median age of 10 months and indicated that PCT was a more robust predictor compared with CRP or WBC count for APN (31). In our hospital, both PCT and ESR require a larger volume of blood samples. We usually acquire blood samples before acquiring urine samples. Because blood sampling is challenging in some young infants, we do not routinely perform these two examinations under an uncertain diagnosis of UTI. Few results were verified in our patients (16 patients in total; 9 in the non-APN group and 7 in the APN group). Now we are still persisting to collect the PCT and ESR data in young infants.
The limitations of our study are its limited study population and retrospective nature. Therefore, additional studies are warranted to evaluate the relationship between APN and inflammatory markers.
5.1. Conclusion
This is the first research on the prediction of APN resulting from febrile UTI specialized in the age group of less than 4 months. In this age group, some factors were influenced by perinatal and prenatal insults. CRP, NLR, and TRT were independent risk factors for APN in our study, but WBC was not related with APN. Only the CRP level had acceptable discrimination for predicting APN in infants less than 4 months old with febrile UTI. If the image survey can’t be arranged timely, CRP can be a parameter to predict the APN. In the age group less than 4 months, we should treat the APN patients longer with antibiotics if CRP exceeds 4.27 mg/dL.