1. Background
Celiac disease is a multifactorial gluten-sensitive autoimmune enteropathy (1). It is recognized in the background of specific HLA-DQ2 or HLA-DQ8 and high levels of antibodies (anti-tissue transglutaminase 2 antibody or Anti-TG2 IgA and anti endomysial antibody or EMA) according to different clinical findings, and in gluten-consuming children according to pathologic findings (2, 3).
Celiac disease is relatively frequent, with the incident rate of about 0.7% - 1.4% in most populations (4). Based on the 2012 ESPGHAN (European Society of Pediatric Gastroenterology, Hepatology and Nutrition) guideline, the diagnosis of celiac disease can be confirmed by pathological changes Marsh II or III in the background of high anti-TG2 ab OR anti-TG2 10-folds above the normal values plus a positive EMA antibody in the presence of HLA-DQ2/HLA-DQ8 in symptomatic children (3).
Hepatitis B is an important health problem in the world (5, 6). Fortunately, an effective and safe vaccine against HBV has been introduced since 1982. Currently, all children are immunized against this infection all over the world (7). The trend of health strategies is to eradicate this infection. A rate of 90% - 100% of vaccinated people whose anti-HBS ab titer is above 10mIU/ml are protected against HBV infection (7, 8).
1.1. Different Factors Are Responsible for Unresponsiveness Against HBV Vaccine
Inappropriate environment of vaccine maintenance, bad site of injection, obesity, cigarette smoking, drug abuse, and some infections. Moreover, alcohol consumption, renal insufficiency, HIV infection, immune suppression, and diabetes mellitus are associated with less antibody production (9-11).
HLA-DQ2 is positive in diseases such as celiac and diabetes mellitus. This HLA is associated with lower titers of HBS ab (12, 13). The gluten consumption during HBV vaccination in celiac-affected children might affect the production of HBS ab (14, 15). Other studies describe that both HBS Ag and gliadin peptides have an affinity for HLA-DQ2; therefore, these two peptides attempt to connect to HLA-DQ2 molecule, resulting in an incomplete antibody response against HBS Ag (15).
These celiac disease-affected children are a significantly large population that can be potentially vulnerable to hepatitis B, and more importantly, they can be a major source of HBV dissemination in the world. This is a critical issue because it can damage the health care system by lowering the vaccination program’s efficiency.
2. Objectives
This study was designed to determine the rate of responsiveness to HBV vaccination (anti-HBS Ab titer > 10 mIU/mL) in a group of Iranian children with celiac disease.
3. Methods
In this cross-sectional case-control study, 31 Iranian children with celiac disease were enrolled as the case group, and 31 age- and sex-matched non-celiac children in the same period were included in the control group.
3.1. Inclusion Criteria
Every child with celiac disease (based on high anti-TG2 ab and duodenal biopsy results, suggesting Marsh II or III, according to our center´s local protocol in the absence of reliable HLA typing laboratories) was included in our case group before introducing a gluten-free diet. Our control group consisted of children without celiac disease, other autoimmune disorders, and well-known immunodeficiency (primary or secondary).
3.2. Exclusion Criteria
Included:
Proven immune deficiency,
Lack of anti-HBV vaccination history,
Use of immunosuppressives during the last three months,
History of transfusion, dialysis, or plasmapheresis during the last three months.
The setting of the study was in Children’s Medical Center, a tertiary level referral hospital in Tehran, Iran. There was no ethical consideration, Anti-HBS Ab test was done free in all patients after obtaining informed consent and assuring confidentiality for the parents/guardians. The serum sample of celiac-affected children before introducing a gluten-free diet and control group children was analyzed for HBS ab titer using the Abbot chemiluminescence kit. Anti-HBS Ab titer above 10 mIU/mL was considered positive. Other data including age, sex, BMI, other concomitant diseases such as diabetes mellitus and autoimmune thyroiditis, time after last HBV vaccination, and anti-TG2 titer were recorded using a questionnaire. We used t-test as well as McNamara and Fisher tests for analysis. The data were analyzed with SPSS software version 20. A P value of less than 0.05 was considered statistically significant.
4. Results
In this study, 31 celiac-affected children aged 24 - 180 months were compared with 31 non-celiac age- and sex-matched children.
4.1. Case Group
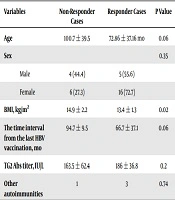
The average age of the cases was 81.84 ± 39.54 months (72 months for boys and 85 months for girls). The HBS ab titer was below 10 mIU/mL in 44.4% of the boys and 27.6% of the girls. However, there was no significant difference between boys and girls (P = 0.35). Moreover, the difference between the boys and girls was not significant, with regard to concomitant autoimmune disease (P = 0.29).
The average age of vaccine responders and non-responders was 72 and 100 months, respectively, and the difference was marginally significant (P = 0.06).
The vaccine responsiveness rate in children younger than 45 months, between 45 - 60 months, between 60 - 114 months, and older than 114 months was 74%, 100%, 70%, and 37.5%, respectively. The Fisher test revealed no significant difference (P = 0.109) in this regard.
Based on the t-test, the time interval from the last HBV vaccination was longer, but not significantly, in the non-responders (94.7 months vs 66.7 months, P = 0.06).
The average level of serum Anti-TG2 Ab in the responders and non-responders was 186 U/mL and 163.5 U/mL, respectively, and the difference was not significant (P = 0.2). The summary of the data is shown in Table 1.
| Variables | Non-Responder Cases | Responder Cases | P Value |
|---|---|---|---|
| Age | 100.7 ± 39.5 | 72.86 ± 37.16 mo | 0.06 |
| Sex | 0.35 | ||
| Male | 4 (44.4) | 5 (55.6) | |
| Female | 6 (27.3) | 16 (72.7) | |
| BMI, kg/m2 | 14.9 ± 2.2 | 13.4 ± 1.3 | 0.02 |
| The time interval from the last HBV vaccination, mo | 94.7 ± 9.5 | 66.7 ± 37.1 | 0.06 |
| TG2 Abs titer, IU/L | 163.5 ± 62.4 | 186 ± 36.8 | 0.2 |
| Other autoimmunities | 1 | 3 | 0.74 |
aValues are expressed as No. (%) or mean ± SD.
4.2. Case and Control
A rate of 67.7% of the cases and 64.5% of the controls had HBS ab titer above 10 mIU/mL. Bi-variable analysis using the McNemar test showed no statistically significant difference (P = 0.73) between the two groups. However, the odds ratio was 0.8, meaning that, although non significantly, celiac disease decreases the antibody production against HBV by up to 20% compared to the control group.
Conditional regression analysis showed that the matched odds ratio for comparing HBS ab titer between the case and control groups was 0.7 (7.26 and 0.07; CI: 95%). After matching for the time interval from the last HBV vaccination, it was observed that although non-significantly, celiac disease can decrease the chance for anti-HBS production up to 30%. The comparison result of the two groups is shown in Table 2.
| Variables | Control Group | Case Group |
|---|---|---|
| Age | 81.87 ± 37.97 | 81.84 ± 39.54 |
| Sex | ||
| Female | 22 (71) | 22 (71) |
| Male | 9 (29) | 9 (29) |
| BMI, kg/m2 | 30.4 ± 16.9 | 13.9 ± 1.76 |
| Responsiveness to HBV vaccine | 20 (64.5) | 21 (67.7) |
| The time interval from the last HBV vaccination, mo | 162 ± 75.71 | 174 ± 75.84 |
| Other autoimmuneities | 0 | 4 (12.9) |
| Diabetes mellitus | 0 | 3 (9.7) |
| Autoimmune thyroiditis | 0 | 1 (3.2) |
aValues are expressed as No. (%) or mean ± SD.
5. Discussion
HBV infection is one of the most important health problems in the world, and vaccination is the best way of prophylaxis (4). The effective blood level of antibody (HBS ab level > 10 mIU/mL) is achieved by a three-dose cycle of HBV vaccination in 95% of children (8). Moreover, 90% - 100% of people with effective HBS ab levels are protected against HBV infection (7).
Moreover, 4% - 10% of vaccinated people do not produce sufficient HBS ab in response to the vaccine, probably because of bad vaccine maintenance conditions, obesity, inappropriate vaccination site (e.g., gluteal), smoking, chronic alcoholism, chronic renal insufficiency, immune deficiency, and other causes (9-11).
HLA-DQ2 is positive in more than 90% of celiac patients (1). This HLA is associated with lower titers of HBS ab (12, 13). Noh et al. (12), in a case-series study in 2003, found that 13 out of 19 celiac patients did not have protective levels of HBS antibodies, and all were HLA-DQ2 positive. Some other studies also showed that antibody production was lower in celiac patients than in non-celiac ones (13, 14, 16-18). Ertekine et al. (17) suggested that there should be a distinct vaccination protocol for celiac patients. Zanoni et al. (18) concluded that these different responsiveness might be related to different intervals from the last HBV vaccination. In our study, this was not confirmed by matching the cases regarding this variable.
Nemes et al. (15) and Leonardi et al. (14) suggested that gluten consumption was a probably important factor in antibody production against the HBV vaccine. However, this was not affirmed in our study, and anti-HBS ab levels were analyzed before introducing a gluten-free diet.
In a systematic review and meta-analysis by Opri et al. (19) in 2015, 12 prospective studies (1447 patients) and four retrospective studies (184 patients) were reviewed. The non-responsiveness rate was 47% and 36% in celiac patients in prospective and retrospective studies, respectively. The difference between celiac and non-celiac patients was statistically significant. They suggested that in celiac disease-affected children, it be better to determine anti-HBS ab levels as soon as possible. Further, they concluded that a personalized anti-HBV vaccination schedule and a follow-up program were necessary for celiac patients (19).
We used a qualitative method to evaluate responsiveness to the HBV vaccine, anti-HBS ab titers more than 10 mIU/mL were considered as responsiveness, which may have affected our study results. Thus, a quantitative study is recommended for assessing the effects of celiac disease on antibody production against HBV vaccine.
5.1. Conclusions
This study did not confirm non-responsiveness against the HBV vaccine in celiac disease in children. This may be due to genetic factors or type of vaccine. Moreover, in our study, responsiveness was assessed qualitatively in the two groups (HBS Ab > 10 mIU/mL was considered as a responder).