1. Background
Surgery and anesthesia are stressful for children. 40% - 60% of children experience a high level of anxiety in the preoperative period. An increase in preoperative anxiety not only causes discomfort for the child and parents, but also leads to delay of induction and recovery after anesthesia. This anxiety causes more pain in the patient and also increases the likelihood of delirium and maladaptive behaviors (1-3). Drug sedation is used for reduction or removal of negative behavior, fear and negative psychological response to treatment, and maximizing amnesia to control behavior during the procedure.
Sedative drugs can be administered by oral, inhalation, rectal, submucosal, intramuscular or intravenous methods. The oral sedation is the simplest and easiest method to manage a sick child who does not cooperate, because there is no need for masking and injection (4). The oral midazolam is the most common pre-anesthetic medicine for children (5). Midazolam is rapidly absorbed from the gastrointestinal tract with peak of action about 30 min and short half-life (1.5 hours) (6).
The oral midazolam at a dose of 0.25 to 0.33 mg/kg usually leads to separation of children from their parents without crying. As an oral pre-med, zolpidem has a short elimination half-life which is on average 2.4 hours in adults compared to diazepam that is 24 - 57 hours (7). Zolpidem is not associated with benzodiazepines in terms of structure, but it seems that its activity, which is associated with activation of GABAergic system, is similar to benzodiazepines (2, 8). Zolpidem is well tolerated in children and is suggested at a dose of 0.25 mg/kg and a maximum dose of 20 mg (9, 10).
2. Objectives
Given the features of these drugs and the need for anxiety control in children, and considering the limited studies conducted in this regard, this research was performed to compare the oral drugs of zolpidem and midazolam on the level of preoperative anxiety in children.
3. Methods
Our study is a prospective double-blind randomized controlled trial. This study was conducted between November 2018 and February 2019 at Rasoul Akram Hospital, Tehran, Iran. According to the study of Bhatnagar (7) the mean sedation score in the zolpidem and midazolam groups was 4.4 ± 0.46 and 4.27 ± 0.85, respectively. The sample size was calculated 23 in each group and 56 in total, with a confidence coefficient of 0.05, a study power of 95%, and according to the following formula:
A total of 56 patients were randomly divided into 2 groups and for randomization of cases we used the RANDBETWEEN command in Excel software, and 56 random codes were allocated (23 for each group). Even codes were allocated to group 1 and odd codes were allocated to group 2. The numbers were decoded after data analysis.
The inclusion criteria for patients were age 3 - 9 years, physically considered in the first or second class based on the American Society of Anesthesiologists (ASA) standard, and undergone the eye examination (looking for retinoblastoma) under general anesthesia in the eye operating room of Rasoul Akram Hospital. The exclusion criteria were contraindication to preoperative sedation (known sensitivity to drugs used in the study and having known metabolic, endocrine, cardiac, pulmonary, and hepatic diseases),decreased level of consciousness, delayed developmental milestones, neurodevelopmental anomalies, hypersensitivity to benzodiazepines and contraindication for midazolam and zolpidem usage, lack of parental consent, weighing below the fifth percentile or above the 95th percentile based on the age and sex-specific growth charts.
At the time of study, 68 patients underwent eye examination under general anesthesia. 12 patients were excluded (due to having exclusion criteria). The weight was measured in fasting state, without shoes and with minimal clothing and using a SECA analog scale with a precision of 0.1 kg. Moreover, height was measured using tape measure with the precision of 0.1 cm and average number of referrals were specified and noted.
Group 1: Patients who received oral midazolam at single dose of 0.25 mg/kg
Group 2: Patients who received oral zolpidem at single dose of 0.25 mg/kg
For masking the bitter taste of drug and for similarity the medicines were dissolved in no-pulp fruit juice. The drug was given to the mother at least 30 minutes before the onset of the induction by a researcher in a paper cup so that preferably the mothers would give it to the patients. The200ml bottles with the same appearance and label, which were blind to the type of drug, were taken to the trial site. Solution 1 contained 0.25 mg/mL of midazolam and solution 2 contained 0.25 mg/ml of zolpidem. Prior to the anesthesia induction, all patients were in fasting state at least two hours for clear liquids, four hours for breast milk, and eight hours for food. In the waiting room before taking the medicine, the level of anxiety in patients was measured. The person who measured the level of anxiety was not aware of the type of drug ingested (double-blind study). The time of drug ingestion was recorded and the child was monitored. Side effects such as paradox effects, agitation, hallucination, respiratory depression, nausea and vomiting were recorded as secondary outcomes. The time elapsed from drug ingestion until separation from parents and the number of referrals (in terms of the difference in anxiety score in those who had the experience of being in the operating room and anesthesia) were recorded in a checklist. Anxiety at the time of entering operating room was measured by the modified Yale Preoperative Anxiety Scale (Appendix 1 in Supplementary File) checklist (11, 12) by one of the members of study team who had practiced administering it in a pilot study conducted before this study. The primary goal of this study was comparison between anxiety score of these two groups in waiting room and at the time of entering the operating room. The Ethics Committee of the Iran University of Medical Sciences approved this study (code IR.IUMS.FMD.REC1396.9411174011) and after complete explanation of study written consent was taken from the parents. The trial was then registered in Iran Clinical Registry (code IRCT20141127020112N7).
3.1. Statistical Analysis
Data was analyzed with SPSS V. 20. The mean and standard deviations were used for descriptive variables, and independent t-test (repeated measure) was used for the relationship between quantitative variables.
4. Results
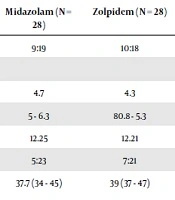
In this study, 70 patients underwent eye examination under general anesthesia. 13 patients were excluded (due to having exclusion criteria). The parents of 57 patients provided the written consent forms. One patient did not undergo general anesthesia because he was diagnosed with acute cold. 28 patients were assigned to the group who received zolpidem, and 28 patients were allocated to the group who received midazolam. 56 patients were enrolled, 19 of them were girls and 37 were boys. The patients’ demographic information and average number of referrals is presented in Table 1.
| Midazolam (N = 28) | Zolpidem (N = 28) | P Valuea | |
|---|---|---|---|
| Sex, F:M | 9:19 | 10:18 | 0.73 |
| Age | 0.28 | ||
| Median | 4.7 | 4.3 | |
| 95% CI | 5 - 6.3 | 80.8 - 5.3 | |
| Weight, mean | 12.25 | 12.21 | 0.54 |
| ASA status 1:2 | 5:23 | 7:21 | 0.46 |
| Average medication consumption time until separation from parents, min | 37.7 (34 - 45) | 39 (37 - 47) | 0.56 |
| Average number of referrals | 4.3 | 4.1 | 0.24 |
aSignificant P value < 0.05.
The anxiety score in the group receiving midazolam was 63.80 (± 12.85) in the pre-test and 32.61 (± 12.6) in the post-test. This decrease was statistically significant (P < 0.001).
The anxiety score in the group receiving zolpidem was 62.49 (± 12.50) in the pre-test and 30.94 (± 12.6) in the post-test. This decrease was also statistically significant (P < 0.001).
The mean score of post-test stress in patients receiving zolpidem was lower than midazolam, and these two are statistically different (P < 0.001).
The level of anxiety in the two groups had no statistical difference before taking medicine (P = 0.420).
In the zolpidem treatment group, 1 (3.6%) patient had epigastric pain and 1 (3.6%) patient had hallucination symptoms and we had no side effects in midazolam group.
5. Discussion
The main purpose of our study was to compare the anxiolytic effects of oral zolpidem and midazolam in children in a double-blind and controlled trial. The results showed that both drugs zolpidem and midazolam reduce preoperative anxiety; however, the preoperative anxiety was significantly decreased after receiving zolpidem in comparison with midazolam. Zolpidem showed a further anxiety reduction 30 minutes after oral intake and had a rapid onset of action. The patient’s cooperation was not disturbed with any of the medicines. Our study showed that oral zolpidem (at a dose of 0.25 mg/kg) had a satisfactory anxiolytic effect before surgery in children.
The drug was given to the patients at a relatively low dose to prevent excessive sedation that might mask the symptoms of anxiety and would not be obvious to others, including researchers collecting information. Therefore, the dose of drug was set to a value less than similar studies (6, 13, 14).
A study conducted by Padhi et al. in 2018 concluded that the most common method to reduce preoperative anxiety was the oral midazolam premedication. The anxiety scores were similar after premedication with midazolam in children with history of anesthesia and those who experienced anesthesia for the first time. However, the overall anxiety was reduced after receiving midazolam (13). The study was conducted only on midazolam and the effects of this medication alone were investigated. The main aim of their study was to measure the anxiety of patients relative to history of previous surgery and they showed that anxiety scores with midazolam premedication were comparable in cases with experience of general anesthesia and those who never had general anesthesia, but they did not specify the amount of midazolam used in their study. In the present study, the number of referrals of patients was measured and evaluated, which was not significantly related to the result of anxiety test and we additionally used zolpidem in our study.
A double-blind study was conducted by Hanna et al. in 2018. It was reported that the effect of midazolam was well accepted. However, there are cases where this medicine is not effective. The mentioned authors compared the effect of zolpidem with midazolam on preoperative anxiety in children. Eighty children of ASA Class I and II, aged 2 - 9 years, were included. Duration of surgery was > 2 hours, and evaluation of patients continued for at least 23 hours after operation. The patients were divided into two groups, one group received 0.5 mg/kg oral midazolam and the other 0.25 mg/kg oral zolpidem. They found that the effects of zolpidem and midazolam on anxiety were not significantly different (14). Our findings are in line with the results of the above study and show the effectiveness of oral zolpidem. It must be noticed that they used 0.5 mg/kg of midazolam, a higher dose than we used, which maybe the cause of obtaining similar effects of midazolam and zolpidem.
In conclusion, our study shows zolpidem as an appropriate anxiolytic premedication in pediatric anesthesia, particularly when the lower anxiety and child co-operation are required for inhalation induction of general anesthesia.
As limitations of our study must be mentioned the not-determining the time required for patients to regain consciousness and not measuring the time spent in recovery. The inclusion of these cases in future studies on zolpidem is suggested.