1. Context
Functional chronic constipation (FC) is a common clinical gastrointestinal disease, and the prevalence of FC in the general population ranges from 15% to 25% (1, 2). FC affects the quality of life of patients of all ages and both sexes. Before 2016, the diagnosis of FC was generally based on the Rome III Criteria for Gastrointestinal Disorders (3). After 2016, the new Rome IV standard introduced a new definition of intestinal-brain interaction disorders for functional gastrointestinal diseases, but there has not been much change in the diagnostic criteria for FC. The new definition emphasizes the development of symptoms associated with dysmotility, visceral hypersensitivity, changing in mucosal and immune functions, changing in gut flora, and abnormal functioning of the central nervous system (CNS).
The cause of FC is mainly related to flora disorders, intestinal motility disorders, pelvic floor dysfunction, psychosocial factors, and gastrointestinal regulatory peptide abnormalities (4). At present, the drugs used in the first-line treatment for FC are mainly various types of laxatives and prokinetic drugs. These drugs have many side effects, and the effects of their long-term use are not ideal (5). However, some FC patients seek alternative therapies due to the lack of beneficial effects and high risk of drug-related adverse events (6). Presently, the use of probiotics in functional gastrointestinal disorders, organic and constipation has gained interest. The change in intestinal flora in chronic constipation provides a theoretical basis for the use of probiotics (7). In fact, flora disorders play a vital role in development of the gastrointestinal disorders such as FC (8, 9).
Probiotics play a beneficial role in intestinal function because they prevent pathogenic bacterial growth via their antimicrobial properties (10). Probiotics also promote intestinal tight junctions and stabilize permeability (11). In addition, probiotics stimulate goblet cells to produce mucus to enhance intestinal barrier function, normalize intestinal peristalsis, and reduce visceral hypersensitivity in children and adults (12).
2. Objectives
There are several studies that suggest a benefit of L. reuteri DSM 17938 in FC (13-17); however, to our knowledge, no systematic reviews have investigated the function of L. reuteri DSM 17938 supplementation in patients with established FC, therefore highlighting the need for further exploration of this topic. The purpose of this meta-analysis was to determine the effects of 4-8 weeks of probiotic supplementation, compared to placebo, on the intestinal movement and fecal consistency in FC patients.
3. Methods
3.1. Data Sources
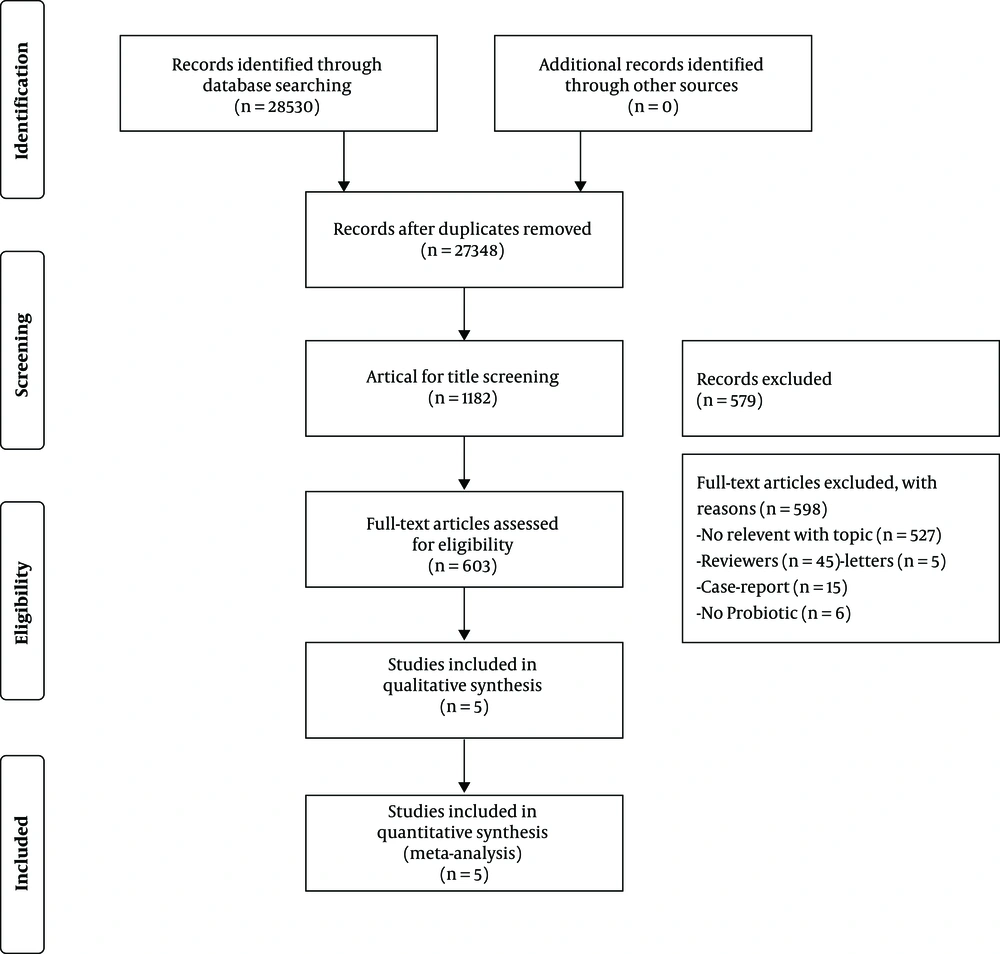
The Embase, PubMed, Ovid MEDLINE, Google Scholar, Web of Science, Cochrane Library, and Clinicaltrials.gov databases were searched to identify articles published between January 2000 and December 2018 (Figure 1). The following search terms were used: (probiotic * OR Lactobacill * OR bacteria * OR ferment * OR microorganism * OR DSM 17938), and (Functional Chronic* OR constipation * OR astriction * OR coprostasis* OR gastrointestinal disease*).
3.2. Study Selection
Two reviewers reviewed the titles and abstracts of all articles. Duplicates, articles with a non-RCT study design, review articles, and articles in which there was no diagnosis of FC or no probiotic intervention were excluded. All relevant features included in the test, such as type of FC, strain of probiotics, dose of probiotics, test and duration of follow-up, and characteristics of the patients, were collected and summarized. All eligible RCT studies considered symptom improvement in FC patients as an outcome of interest. The reference lists of articles identified from the search were reviewed to identify any other eligible articles. Characteristics of the included articles (13-17) are shown in Table 1.
| Study | Country | Criteria | Number(Probiotics /Placebo) | Age | Sex (male) | Treatment | Design | Supplementation | Main Findings |
|---|---|---|---|---|---|---|---|---|---|
| Flavia Indrio (2014) (14) | Italy | Rome III | 238/230 | 90 days | 122/120 | 12 weeks | RCT | 1×108 colony-forming units of L. reuteriDSM17938 | Daily administration of L reuteri DSM 17989 early in life decreased the reported incidence of inconsolable crying, regurgitation, and functional constipation in the first 3 months of life. |
| Veronica Ojetti (2014) (17) | Italy | Rome III | 20/20 | 35.6±15 years | 9/7 | 4 weeks | RCT | 2×108 colony-forming units of L. reuteri DSM17938 | The supplementation with L. reuteri (DSM 17938) signicantly improved bowel movements, increasing the frequency of evacuations per week in adult patientsaected by chronic functional constipation. |
| Agnieszka Wegner (2018)16) | Poland | Rome III | 65/64 | 3 - 7 years | 29/28 | 8 weeks | RCT | 1×108 colony-forming units of L. reuteri DSM17938 | L. reuteri DSM 17938 supplementation as an additional therapy to macrogol did not have any beneficial effect on the treatment of functional constipation in children aged 3 - 7 years. |
| Paola Coccorullo (2010) (13) | Italy | Rome III | 22/22 | 8.2 ± 2.4 years | 8/16 | 8 weeks | RCT | 1×108 colony-forming units of L. reuteriDSM17938 | L. reuteri DSM 17938 supplementation was effective in increasing bowel movements |
| G. Riezzo (2017) (15) | Italy | Rome III | 28/28 | 19 - 65 years | 4/1 | 105 days | RCT | 7×108 colony-forming units of L. reuteri DSM17938 | L. reuteri DSM 17938 seems to control symptoms other than stool consistency, more related to gas production and dysbiosis |
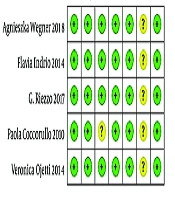
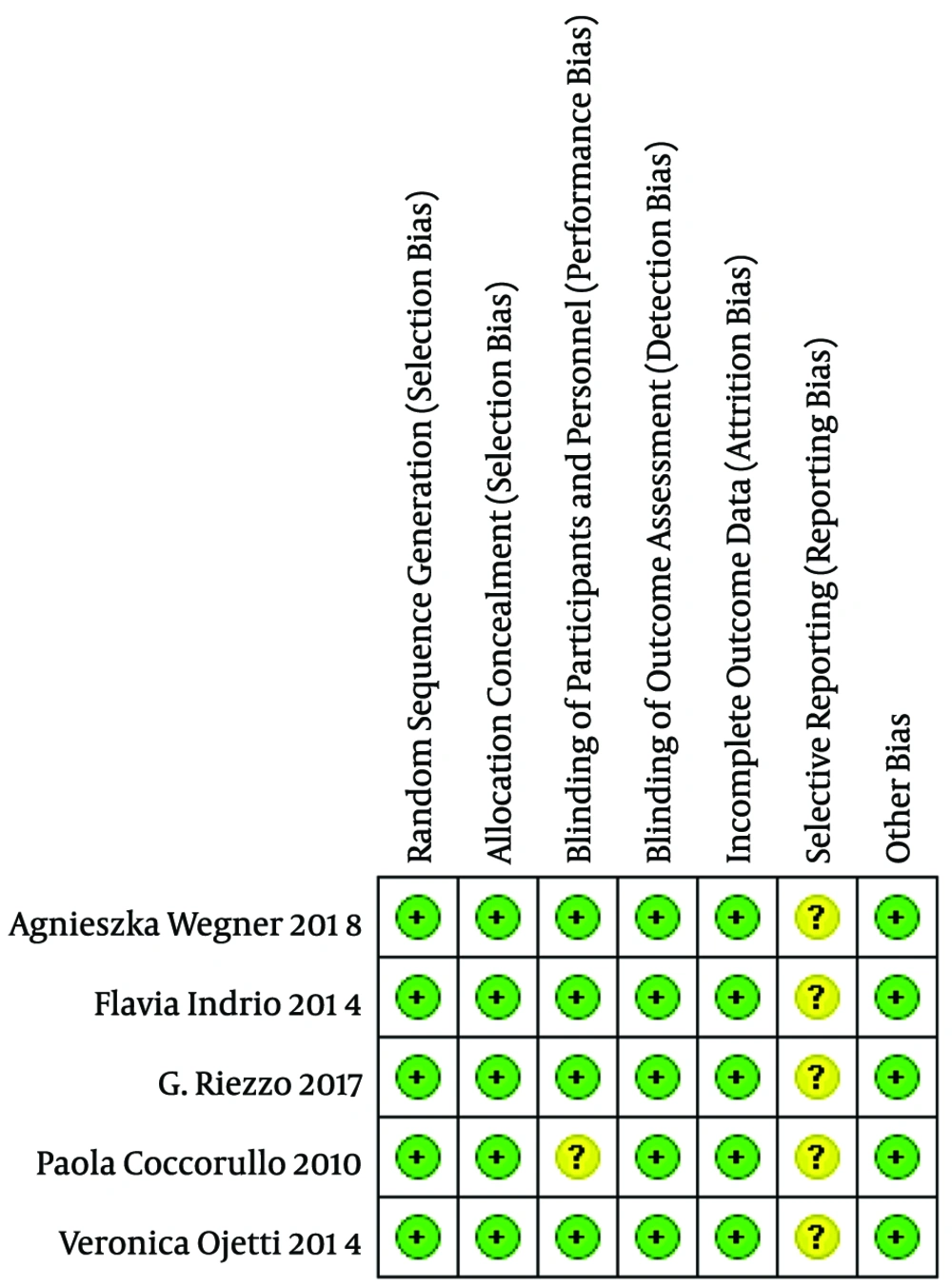
3.3. Assessment of Trial Quality
All the studies included in the review had a low risk of bias. The Jadad score, which indicates the quality of all studies based on the description of selection, performance, detection, attrition, and reporting and other bias, was used to evaluate the methodological quality of the trials (Figure 2).
3.4. Assessment of Risk of Bias in Included Studies
The risk of bias was assessed by two independent review authors (XW and RY), and disagreements were resolved through consultation with a third review author (XL). We used the Cochrane Collaboration tool to assess the risk of bias in each of the included studies. Based on our judgments on each area or potential bias risks, we assigned the study as having one of three categories: “low risk of bias”, “unclear risk of bias” or “high risk of bias”. Whether the study should be included in the meta-analysis was determined from a separate assessment based on the outcome of the bias assessment risk, and studies considered to have high risk of bias were excluded. The articles that were eventually included in the meta-analysis were judged by XW and XL and supervised by RY based on the results of the risk of bias assessment.
4. Results
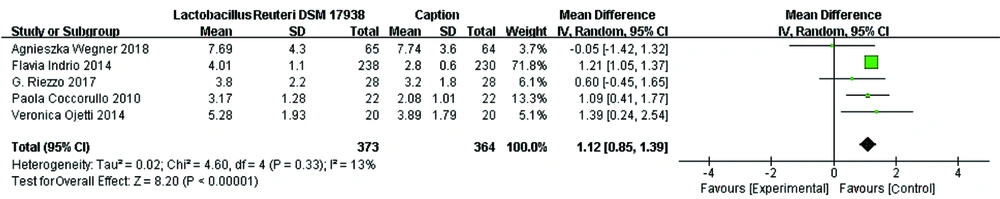
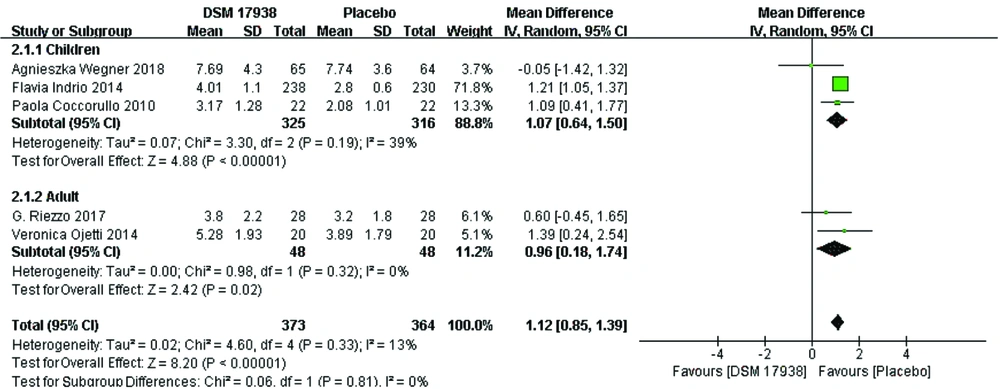
Bowel movements: Five studies investigated the effect of L. reuteri DSM 17938 supplementation on bowel movements in patients with FC. Four (13-15, 17) of the five studies reported a statistically significant increase in bowel movements in the groups receiving L. reuteri DSM 17938 in comparison to the groups receiving placebo. The meta-analysis of all five trials (n = 737) indicated a significant increase in bowel movements in FC following L. reuteri DSM 17938 supplementation (mean difference = 1.12; 95% CI 0.85, 1.39, P < 0.00001). In addition, the results of our meta-analysis showed low between-study heterogeneity (df = 4, P = 0.33, I2 = 13%) (Figure 3).
Subgroup Analysis of bowel movements by Age: The five trials involved patients aged 0–65 years. We categorized the patients into two groups: children 0-18 years old and adults >18 years old. Accordingly, three trials (14, 16, 17) were included in the 0-18 years old subgroup, and two trials (13, 15) were included in the >18 years old subgrou The efficacy of L. reuteri DSM 17938 in the former subgroup was 1.07 (95% CI 0.64, 1.50, P < 0.00001) and that in the latter subgroup was 0.96 (95% CI 0.18, 1.74, P = 0.02). Moreover, a low degree of heterogeneity was observed between the two subgroups (Figure 4).
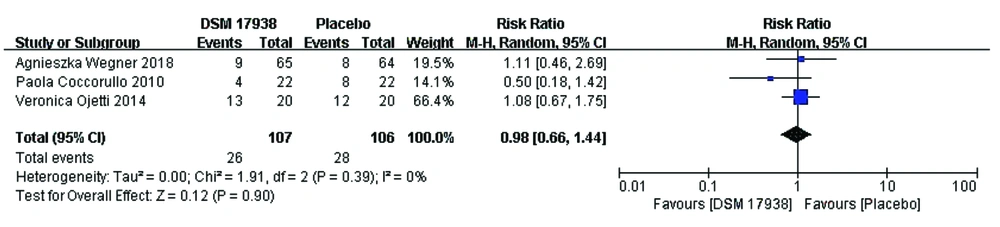
Stool consistency: Three trials (13, 16, 17) evaluated stool consistency to compare the effect of L. reuteri DSM 17938 therapy with that of placebo in FC patients. However, there was no change in fecal consistency after FC patients were supplemented with probiotics or placebo. No significant differences in stool consistency were observed following L. reuteri DSM 17938 supplementation (0.98; 95% CI 0.66, 1.44, P = 0.90). In addition, the results of our meta-analysis showed low between-study heterogeneity (df = 2, P = 0.39, I2 = 0%) (Figure 5).
5. Discussion
Our analysis suggests a benefit of probiotic supplementation in patients with FC; age-specific subanalyses showed that probiotics effectively increased bowel movements in both children aged 0–18 years and adults aged >18 years. However, no significant differences in stool consistency were observed following probiotic supplementation.
In a previous study, researchers have found that levels of Clostridium and Bifidobacterium bacteria in stool of children with constipation were significantly higher than those of healthy children (18). Subsequently, Khalif cultured the intestinal flora of adult patients with functional constipation and found that the Bifidobacterium and L. levels were significantly reduced in the stool samples compared to those without FC and that the microbial changes in the stool of patients with severe constipation are more obvious (19). The current research found that the intestinal flora may play an important role in the occurrence and development of functional constipation (20). Constipation can lead to an imbalance of intestinal flora, and unbalanced intestinal flora can have many adverse effects on human health, such as the production of a variety of enterotoxin, development of colon cancer, acceleration of aging and promotion of a variety of intestinal diseases (21). Compared with the intestinal flora of normal people, the intestinal flora of patients with functional constipation is different, which is mainly due to the relative reduction in obligate anaerobic bacteria and the relative increase in potentially pathogenic bacteria (22-24). In addition, intestinal flora imbalance leads to abnormalities in itself and its metabolites, such as a decrease in short-chain fatty acids and an increase in the production of gases such as methane. In turn, it affects the changes of related signal pathways, leading to intestinal-brain axis imbalance, abnormal intestinal motility, and eventually constipation.
Probiotics used to treat constipation started when researchers found that the intestinal microecology of constipation was different from the normal intestinal microecology (25). No adverse reactions of probiotics were found in these studies. Probiotics are low-pathogenic or nonpathogenic microorganisms that are mainly planted in the human intestines and reproductive system (26). They are beneficial for the host and are made from a single strain or a combination of multiple strains of bacteria. The mechanism of probiotics in treating functional constipation may be through the stimulation of cholecystokinin and deconjugation of bile salts, resulting in free secondary bile salts that can stimulate colonic motility or lower colonic pH, and through the production of other short-chain fatty acids, which may stimulate peristalsis (27-29).
At present, the study of probiotics for the treatment of functional constipation is usually based on the number of weekly bowel movements, the total score of constipation-related symptoms and the total score of fecal traits to describe the therapeutic effect of probiotics (30). This study found that probiotics have different therapeutic effects in patients with functional constipation rather than what they do in healthy patients, which may be due to the small sample size, different experimental design methods performed, different probiotic strains used, different doses and different durations of administration.
An important advantage of our analysis is that it focuses on a well-defined single bio-probiotic microorganism, L. reuteri DSM 17938. Thus, our results precisely defined the effects of the administration of L. reuteri DSM 17938 in patients with FC. Decreased number of bowel movements is one of the important diagnostic criteria for functional constipation, and the increase in the number of bowel movements after oral probiotics is not only a manifestation of the relief of functional constipation, but also helps to alleviate anxiety and improve the quality of life of patients, it has important clinical implications.
However, there are also many limitations to this analysis. Gray literature was not retrieved, and the search language was limited to Chinese and English. There was also variation in not only the number of trials but also the sample sizes in the trials. There are few criteria used for evaluating the effect of L. reuteri DSM 17938 for FC, but this study included only the number of bowel movements and stool consistency. In addition, it should be stated that although Riezzo's research lasted for 105 days, Flavia's research lasted for 12 weeks, we still included those in the statistics. It is because their experiments have produced clinical effects in 4-8 weeks, and there is no significant difference between the 12th week and the week before.
6. Conclusions
Our meta-analysis provides evidence that L. reuteri DSM 17938 supplementation improves stool frequency but not stool consistency in patients of all ages. For FC patients, the increased frequency of defecation may largely alleviate the pain of defecation. The results demonstrate the beneficial effects of L. reuteri DSM 17938 in functional chronic patients. More adequately powered RCTs are needed to determine which doses and duration of L. reuteri DSM 17938 are efficacious in FC treatment. Moreover, the need to study the mechanisms of action of L. reuteri DSM 17938 is warranted.