1. Background
Free radicals (FR) are extremely reactive and unstable molecules due to presence of an unpaired electron in outermost orbit (1). Oxygen containing FRs are named as reactive oxygen species (ROS). Oxidative stress occurs when there is imbalance between ROS production and anti-oxidant capacity. Redox cascade initiated by immune activation involves production of considerable amount of ROS and reactive nitrite species (RNS), activation of DNA transcription process and finally lethal mitochondrial dysfunction and multiorgan failure (2). Endogenous antioxidants are superoxide dismutase (SOD), catalase, glutathione peroxidase, glutathione reductase, perioxyredoxin, thioredoxinreductase, glutathione, flavonoids, biluribin, uric acid, melatonin, thiols, reduced coenzyme Q, alpha lipoic acid, endogenous organic selenium, metal binding proteins transferrin, ferritin, lactoferrin, ceruloplasmin and albumin (3).
Thiols are organic molecules and analogues of sulfide alcohols. Thiols (RDH), by formdisulphide bonds (RSSR) may take place in oxidation reactions (4). Majority of plasma thiol pool is formed predominantly by albumin and other proteins, whereas minority is formed by low molecular thiols such as csystein, glycin containing cystein, glutathion, homocystein and gama-glutamylcystein. Thiol groups of proteins and low molecular weight thiols are oxidized by available oxidant molecules and are converted to tertiary disulphide bond structures. Formed disulphide bonds may be reduced to thiol groups and thioldisulphide balance is maintained (5).
Thiol and dynamic disulphide molecules take place in many pathways including stabilization and regulation of proteins/enzymes, receptors and Na/K channel transport. Dynamic thiol/disulphide balance has critical role in antioxidant defence, detoxification, apoptosis, transcription and cellular signal transport mechanisms (6, 7). This balance is also documented to take place in pathogenesis of diabetes mellitus, cardiovascular diseases, cancer, rheumatoid arthritis, chronic renal insufficiency, Parkinson disease, Alzheimer’s disease, multiple sclerosis and hepatic diseases. The dynamic thioldisulphide balance can be measured by a calorimetric method (8).
Oxidative stress is a pathogenic stress factor in preterm infants. Oxidant/antioxidant balance state is a process beginning before birth and premature infants are especially vulnerable to oxidative stress. Complications of prematurity like bronchopulmonary dysplasia (BPD), retinopathy of prematurity (ROP) and necrotizing enterocolitis (NEC) are related with oxidative stress, mainly production of ROS and imbalance between antioxidant capacity of the newborn (9, 10).
2. Objectives
In this study, we aimed to evaluate dynamic thiol/disulphide homeostasis by umbilical blood gas analysis, compare term and preterm infants, evaluate its relation with Apgar score and occurrence of NEC, sepsis and ROP.
3. Methods
3.1. Study Group
A total of 108, 51 female and 57 male newborn infants, were included in this prospective cohort study conducted between June 2018 and June 2019. Of them, 31 were term (> 37 weeks) and 77 were preterm (< 37 weeks). Patients with congenital heart defects, metabolic diseases and other congenital anomalies were excluded. The study was carried out in a hospital with a tertiary NICU. The study protocol was approved by Local Ethical Committee and informed consent was obtained from the parents. The study was conducted in accordance with Declaration of Helsinki, Basil Protocol 2013. Demographic variables, Apgar score, NEC, sepsis and ROP intervention data were collected.
3.2. Method
Two ml of umbilical venous samples of term and preterm infants were collected for thiol/disulphide homeostasis tests and serum was separated by centrifuging at 1500 rpm for 10 minutes. Serum samples were stored at -80°C. Serum total ischemic modified albumin (IMA) and albumin were measured by biochemical analysis. Native thiol and total thiol levels were measured as instructed by Erel and Neselioglu (11) in 2014 followed by calculation of disulphide, disulphide/native thiol, disulphide/total thiol and native thiol/total thiol ratios.
Briefly, reducible disulphide bonds were first reduced to form free functional thiol groups. Unused reductant sodium borohydride was consumed and removed with formaldehyde, and all thiol groups including reduced and native ones were detected after reaction with five, 5’-dithiobis-(2-nitrobenzoic) acid. Half of the difference between total and native thiols provided the dynamic disulfide amount. Disulfide to native thiol ratio, disulfide to total thiol ratio, and native thiol to total thiol ratio were calculated and were presented as percentage. Ratios were depicted as index 1, 2, and 3 and are as follow:
Disulphide: (total thiol-native thiol)/2
Index 1: Disulphide/native thiol × 100
Index 2: Disulphide/total thiol ×100
Index 3: Native thiol/total thiol ×100
Units were presented as: Albumin-g/dl; IMA-ABSU; native thiol-μmol/l; total thiol-µmol/L.
From every sample collected, blood gas analysis was performed within 2 minutes following birth (Radiometer ABL 800 FLEX). pH value was measured potentiometically. Umbilical cord blood pH values were divided into three groups in both term and preterm infants; pH between 7.21 and 7.30 as normal, pH between 7.21 and 7.0 as acidotic and pH < 7.0 as asphyxic (12). Patients were divided into two based on Apgar scores: < 7 and ≥ 7.3
3.3. Statistical Analysis
Statistical analyses were performed using SPSS software for Windows version 22.0 (Statistical Package for the Social Sciences Inc, Chicago, IL, USA). Distribution of normality was measured by Shapiro-Wilk test. Continuous variables were expressed as mean values ± standard deviation (SD). Characteristics were compared using Student t-test for continuous variables. For evaluation of relation between maternal age and albumin, IMA, native thiol, disulphide and total thiol Mann-Whitney U-test was used. Statistical significance was set as P < 0.05. For all comparisons with a statistically significant minimum P < 0.05 level, effect sizes and post hoc power analysis were performed in G* Power software. In the study, the minimum and maximum effect sizes were calculated as 0.406 - 0.752. The strength of the test was calculated as minimum 64.2% and maximum 90.5%.
4. Results
Thirty-one term (28.7%) (≥ 37 weeks) and 77 preterm (81.3%) (< 37 weeks) were included in the study in whom 51 were female (47.2%) and 57 were male (52.8%). C/section was performed in 105 cases whereas normal vaginal delivery in 3.
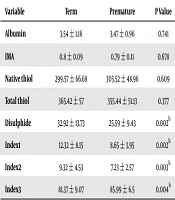
When albumin, IMA, disulphide, total thiol and native thiol levels and index 1, 2, and 3 ratios were compared between term and preterm infants, preterm infants had lower disulphide levels and index 1 and 2 ratios, but higher index 3 ratios (P < 0.05 for each) (Table 1). Minimum and maximum effect sizes were calculated as 0.585 - 0.630 in comparisons of mature and premature groups in terms of albumin, IMA, disulphide, total thiol and native thiol levels and index 1, 2, and 3 ratios. For the same comparisons, the strength of the test was calculated as minimum 86.2% and maximum 90.3%.
| Variable | Term | Premature | P Value |
|---|---|---|---|
| Albumin | 3.54 ± 1.16 | 3.47 ± 0.96 | 0.741 |
| IMA | 0.8 ± 0.09 | 0.79 ± 0.11 | 0.678 |
| Native thiol | 299.57 ± 66.68 | 305.52 ± 48.96 | 0.609 |
| Total thiol | 365.42 ± 57 | 355.44 ± 51.13 | 0.377 |
| Disulphide | 32.92 ± 13.73 | 25.59 ± 9.43 | 0.002b |
| Index1 | 12.32 ± 8.15 | 8.65 ± 3.95 | 0.002b |
| Index2 | 9.32 ± 4.53 | 7.23 ± 2.57 | 0.003b |
| Index3 | 81.37 ± 9.07 | 85.99 ± 6.5 | 0.004b |
aValues are expressed as mean ± SD.
bP < 0.05.
Twenty-two patients had acidotic (20.3%) and 86 had normal (79.7%) blood pH; of 22 acidotic infants, 10 were preterm and 12 were term. Among 86 patients with normal pH, 67 were premature and 19 were term. When IMA, disulphide, total thiol and native thiol levels and index 1, 2, and 3 ratios were compared in acidotic infants, index 1 and 2 and disulphide levels were higher and index 3 was lower than patients with normal pH (P < 0.05). When normal premature infants and acidotic premature infants were compared, there were no statistically significant differences. Impact size: 0.547 - 0.678, power 67.1% - 82.7% (Table 2).
| Variable | Normal | Acidotic | ||||
|---|---|---|---|---|---|---|
| Mature | Premature | P Value | Mature | Premature | P Value | |
| Albumin | 3.27 ± 1.09 | 3.48 ± 0.95 | 0.413 | 3.98 ± 1.18 | 3.42 ± 1.13 | 0.277 |
| IMA | 0.81 ± 0.09 | 0.79 ± 0.11 | 0.485 | 0.78 ± 0.09 | 0.76 ± 0.13 | 0.824 |
| Native thiol | 305.56 ± 68.53 | 305.76 ± 42.59 | 0.987 | 290.09 ± 65.45 | 303.93 ± 83.28 | 0.667 |
| Total thiol | 365.65 ± 58.28 | 354.23 ± 46.61 | 0.376 | 365.05 ± 57.47 | 363.54 ± 77.87 | 0.959 |
| Disulphide | 30.04 ± 11.44 | 24.96 ± 8.3 | 0.034b | 37.48 ± 16.23 | 29.81 ± 14.92 | 0.266 |
| Index 1 | 11.07 ± 7.53 | 8.32 ± 3.11 | 0.020b | 14.31 ± 9.02 | 10.89 ± 7.39 | 0.349 |
| Index 2 | 8.57 ± 4.22 | 7.05 ± 2.13 | 0.034b | 10.49 ± 4.94 | 8.45 ± 4.52 | 0.327 |
| Index 3 | 82.86 ± 8.44 | 86.43 ± 6 | 0.041b | 79.01 ± 9.88 | 83.1 ± 9.03 | 0.327 |
aValues are expressed as mean ± SD.
bP < 0.05.
Forty-eight patients (44.4%) were taken to NICU whereas 60 not (55.6%). Disulphide, index 1 and 2 were lower in patients taken to NICU compared to ones who were not, whereas index 3 values were higher (P < 0.05), Impact size: 0.406 - 0.526, power 66.9% - 85.4% (Table 3).
| NICU | 5th Minute Apgar | |||||
|---|---|---|---|---|---|---|
| Patients Taken to NICU (N = 48) | Patients Not Taken to NICU (N = 60) | P Value | < 7 (N = 21) | > 7 (N = 87) | P Value | |
| Albumin | 3.52 ± 0.94 | 3.47 ± 1.08 | 0.783 | 3.55 ± 0.88 | 3.48 ± 1.05 | 0.760 |
| IMA | 0.79 ± 0.13 | 0.79 ± 0.09 | 0.853 | 0.79 ± 0.13 | 0.79 ± 0.1 | 0.850 |
| Native thiol | 306.25 ± 53.35 | 301.87 ± 55.55 | 0.679 | 307.17 ± 48.35 | 303 ± 55.96 | 0.754 |
| Total thiol | 353.26 ± 53.23 | 362.33 ± 52.56 | 0.377 | 350.28 ± 53.05 | 360.24 ± 52.87 | 0.441 |
| Disulphide | 24.53 ± 8.95 | 30.23 ± 12.33 | 0.008b | 23.88 ± 9.64 | 28.62 ± 11.49 | 0.084 |
| Index 1 | 8.45 ± 4.81 | 10.71 ± 6.18 | 0.040b | 8.01 ± 4.14 | 10.12 ± 5.96 | 0.129 |
| Index 2 | 7.03 ± 2.86 | 8.46 ± 3.63 | 0.028b | 6.81 ± 2.63 | 8.07 ± 3.49 | 0.123 |
| Index 3 | 86.65 ± 7.59 | 83.08 ± 7.25 | 0.014b | 88.03 ± 9.11 | 83.85 ± 6.98 | 0.023b |
aValues are expressed as mean ± SD.
bP < 0.05.
When albumin,IMA, disulphide, total thiol and native thiol levels and index 1, 2, and 3 ratios were compared based on Apgar scores, 21 patients (19.4%) with scores < 7 had higher index 3 values at 5th minute (P < 0.05), Impact size: 0.406 - 0.526, power 66.9% - 85.4% (Table 3).
Nineteen patients had the diagnosis of NEC (17.6%). All of our 19 patients with the diagnosis of NEC were premature and 14 of them were evaluated as Suspected necrotizing enterocolitis and 5 of them were Definitive medical necrotizing enterocolitis according to the modified bell criteria (13). Disulphide levels, index 1 and 2 levels were lower in patients with NEC and index 3 levels were higher (P < 0.05). There was no statistical difference in total thiol and native thiol levels, Impact size: 0.554 - 0.752, power 70.3% - 90.5% (Table 4).
| NEC | ROP | |||||
|---|---|---|---|---|---|---|
| NEC (N = 19) | NO NEC (N = 89) | P Value | ROP (N = 18) | NO ROP (N = 90) | P Value | |
| Albumin | 3.73 ± 0.91 | 3.44 ± 1.04 | 0.227 | 3.62 ± 0.98 | 3.47 ± 1.03 | 0.566 |
| IMA | 0.76 ± 0.14 | 0.80 ± 0.10 | 0.338 | 0.80 ± 0.12 | 0.79 ± 0.10 | 0.570 |
| Native thiol | 322.44 ± 50.88 | 299.84 ± 54.54 | 0.094 | 304.56 ± 40.61 | 303.67 ± 56.91 | 0.950 |
| Total thiol | 362.76 ± 57.06 | 357.35 ± 52.15 | 0.707 | 350.47 ± 41.91 | 359.87 ± 54.79 | 0.493 |
| Disulphide | 22.73 ± 6.20 | 28.76 ± 11.84 | 0.003b | 25.67 ± 10.82 | 28.1 ± 11.38 | 0.406 |
| Index1 | 7.18 ± 2.14 | 10.24 ± 6.08 | 0.000b | 8.76 ± 4.72 | 9.89 ± 5.88 | 0.442 |
| Index2 | 6.32 ± 1.59 | 8.15 ± 3.56 | 0.001b | 7.33 ± 2.97 | 7.93 ± 3.45 | 0.492 |
| Index3 | 89.19 ± 8.21 | 83.7 ± 7.12 | 0.012b | 87.27 ± 10.2 | 84.14 ± 6.9 | 0.110 |
aValues are expressed as mean ± SD.
bP < 0.05.
Eighteen patients had ROP (16.6%). The diagnosis of ROP of 18 premature patients was made according to ICROP (International Classification of Retinopathy of Prematurity) as a result of routine eye examination performed in our clinic. 12 patients stage 1: Demarcation line separating vascular and avascular retina, 4 patients stage 2: Back (ridge). The surface was swollen and 2 patients reported as stage 3: Extraretinal fibrovascular proliferation at the back (14). There were no statistically significant differences in IMA, disulphide, total thiol and native thiol levels and index 1, 2, and 3 ratios between ones with or without ROP, Impact size: 0.554 - 0.752, power 70.3% - 90.5% (Table 4).
Twenty-six patients (24.1%) had neonatal sepsis. Patients with positive blood, CSF and urine cultures (> 72 hours) were diagnosed as sepsis (15). When IMA, disulphide, total thiol and native thiol levels and index 1, 2, and 3 ratios were compared, index 3 ratios were higher in septic infants (P < 0.05), Impact size 0.455 and power 64.2% (Table 5).
| Sepsis | P Value | ||
|---|---|---|---|
| Sepsis (N = 26) | No Sepsis (N = 82) | ||
| Albumin | 3.51 ± 0.87 | 3.48 ± 1.06 | 0.911 |
| IMA | 0.8 ± 0.12 | 0.79 ± 0.1 | 0.783 |
| Native thiol | 306.47 ± 45.92 | 302.97 ± 57.03 | 0.777 |
| Total thiol | 352.24 ± 48.92 | 360.23 ± 54.12 | 0.504 |
| Disulphide | 24.77 ± 9.26 | 28.63 ± 11.74 | 0.129 |
| Index1 | 8.33 ± 3.97 | 10.14 ± 6.1 | 0.160 |
| Index2 | 7.05 ± 2.51 | 8.07 ± 3.58 | 0.176 |
| Index3 | 87.24 ± 8.44 | 83.85 ± 7.15 | 0.047* |
aValues are expressed as mean ± SD.
bP < 0.05.
Two mothers had eclampsia (1.8%) and 8 had preeclampsia (7.4%). Index 3 was higher in patients born from preeclamptic mothers compared to ones without preeclampsia (P < 0.05) (Table 6).
| Preeclamptic Mother | P Value | ||
|---|---|---|---|
| Preeclampsia (N = 8) | No Preeclampsia (N = 100) | ||
| Albumin | 3.46 ± 0.48 | 3.49 ± 1.05 | 0.760 |
| IMA | 0.78 ± 0.11 | 0.79 ± 0.11 | 0.850 |
| Native thiol | 325.11 ± 41.23 | 302.11 ± 55.1 | 0.754 |
| Total thiol | 360.8 ± 55.46 | 358.1 ± 52.88 | 0.441 |
| Disulphide | 23.96 ± 4.09 | 28 ± 11.62 | 0.084 |
| Index 1 | 7.41 ± 1.23 | 9.89 ± 5.88 | 0.129 |
| Index 2 | 6.66 ± 0.81 | 7.92 ± 3.48 | 0.123 |
| Index 3 | 91.01 ± 12.02 | 84.16 ± 6.96 | 0.023b |
aValues are expressed as mean ± SD.
bP < 0.05.
There were 12 (11.1%) infants with Rh incompatibility. Total thiol values were higher in patients with Rh incompatibility (P < 0.05).
Here were 5 infants (4.6%) diagnosed with prenatal fetal distress. There were not statistically significant differences in IMA, albumin, disulphide, total thiol and native thiol levels and index 1, 2, and 3 ratios in patients with or without prenatal fetal distress.
Seven placental anomalies were observed among mothers (6.4%). Albumin levels were higher in patients with maternal placental anomalies (P < 0.05).
Only 6 mothers were smokers (5.5%). There were no statistically significant differences in thiol, disulphide, total thiol and native thiol levels between smoker and non-smoker mothers’ infants. Ten patients had gestational diabetes (9.2%) and thiol, disulphide, total thiol and native thiol levels, index 1, 2, and 3 levels did not differ between patients with maternal gestational diabetes or not.
5. Discussion
Oxidative stress has been defined as a risk factor for occurrence of abortus, preeclampsia and intrauterine growth retardation and future preterm births. Bioparameters of oxidative stress were reported to be early diagnostic markers in high risk patients (16). It is possible to evaluate oxidative stress and antioxidant balance by evaluating cord blood thiol homeostasis.
To the best of our knowledge, our study is the first to evaluate the relationship between infant cord blood dynamic thiol-disulfide balance with perinatal risk factors and various newborn diseases. In preterm infants, early cessation of placental-fetal antioxidant transfer and insufficient synthesis of endogenous production lead to improper antioxidant capacity (17). Moreover, the newborn is born to an environment of 100 mmHg pO2 compared to rather hypoxic intrauterine environment (pO2 of 15 - 20 mmHg). This also increases oxidant stress (18).
In our study, we documented a rather increased antioxidant thiol, native and total thiol levels than disulphide ratios in preterm infants compared to term ones. Unal et al. (19) studied dynamic thiol homeostasis in VLBW infants at birth and 1 and 3 weeks after birth. Native and total thiol levels were increased from birth to first week and from first to third week whereas disulphide ratios were increased at firs week followed by a decrease in third (19). They have documented higher native and total thiol levels in preterm infants similar to our study. Increase in oxygen consumption after birth leads to ROS and physiologic oxidative stress. Expression of antioxidant enzymes like SOD, catalase and glutathione peroxidase changes dynamically in late gestational weeks when fetal lungs are prepared for respiration. Most important non-enzymatic antioxidants like reduced GSH, thyroredoxin, hemooxygenases, vitamins C and E, beta karoten and transient metal chelators are not available till the end of pregnancy. Overproduction of FRs exceeds antioxidant capacity especially in preterm infants. This vulnerability to oxidative injury increases FR related disease risk that begins in prenatal period (20). We may explain the higher antioxidant thiol, native and total thiol levels compared to disulphide in cord blood of preterm infants as a compensatory mechanism. This may be a protective strategy against FR injury related with prematurity that begins in prenatal period and continues with resuscitation and mechanical ventilation and hence may be a surrogate of earlier maturation of thiol pool compared to other antioxidant mechanisms.
During reperfusion, one of the most important FRs causing cellular damage is produced while xanthine dehydrogenase (XD) is converted to xanthine oxidase (XO). Xanthine dehydrogenase utilizes NADPH as an electron recipient, but cannot transfer electrons to molecular oxygen. Conversion of XD to XO involves oxidation of thiol groups or proteolysis by proteases for Ca transport in energy depleted cells (21). As we have documented, low cord blood disulphide levels and index 1 and 2 ratios in preterm infants may increase reperfusion and are similar to recent literature.
In our study, index 3 values were higher in infants born from preeclamptic mothers compared to ones without. In study by Korkmaz et al. (22), while the severity of preecmplsia increased, native and total thiol levels were increased, whereas ratio of them (index 3) was not compared to non-preeclamptic mothers. This may be related with lower placental transfer of total thiol in their study, whereas lower number of preeclamptic patients in our study. Bharadwaj et al. (23) also documented higher oxidative stress markers in preeclamptic mothers and increased protein carbonyl levels in cord blood. Our study also revealed increased oxidative stress determined by increased index 3.
We have documented increased index 3 ratios in 26 infants with sepsis. Increased native thiol levels compared to total may be suggestive for increased antioxidant capacity in neonatal sepsis. However, Aydogan et al. (24) observed decreased native and total thiol levels, increased disulphide/total thiol ratios and decreased native thiol/total thiol ratios.
In pathogenesis of NEC, ROS, and FRs are very important for disruption of intestinal barriers (25). In animal studies, it has been proposed that in NEC, pathogenesis is related with decreased capacity of the neonatal gut epithelial cells (NGECs) to overcome OS during enteral feeding, and an artificial model revealed this impaired capacity results in apoptosis and inflammation of NGECs (26). In our study, in preterm infants disulphide levels and index 1 and 2 ratios were lower and index 3 levels were higher in thiol homeostasis. This reveals an increased, antioxidant ratio leading to increased prenatal oxidant stress and compensatory antioxidant stress that further predispose to NEC. This may be further verified in studies with higher number of patients.
We have observed that index 3 ratios were higher in patients with an Apgar score of < 7. This may be due to a higher increase in native thiol levels compared to total thiol induced by oxidative stress that begins in intrauterine period. Sinharay et al. (27) examined the relation between oxidative markers methemoglobin, erythrocyte glucose phosphate dehydrogenase, erythrocyte GSH and Apgar score and observed that patients with lower scores were exposed to more oxidative and nitrosative stress and had lower antioxidant levels. Noh et al. (28) examined lipid peroxide and malonyldialdehyde levels in cord blood and did not conclude in significant differences in different Apgar score values and explained this situation with low number of patients studied.
We studied 48 patients taken to NICU and they had lower disulphide levels and index 1 and 2 ratios and higher index 3 ratios compared to ones not taken. We were not able to document an estimated increase following intervention in NICU since we did not make further analysis afterwards. Kapadia et al. (29) studied oxidative stress biomarkers in preterm infants stabilized with 100% oxygen or air. Total hydroperoxide (TH), biological antioxidant potential (BAP) and TH/BAP were studied in cord blood and one hour after birth. The ratio was higher in infants stabilized with air on first hour. We also believe that studies evaluating preterm thiol homeostasis would be useful following intervention.
The present study has some limitations. The numbers of premature and term babies in the patient group studied in the article are not equal. In addition, NEC and ROP are diseases belonging to premature babies. In our study, as a limitation the statistical evaluation includes the total number, we cannot study subgroups due to the limited number of cases. Other limitation is, due to the low number of patients belonging to perinatal risk groups, the changes in native thiol, total thiol, disulphide, index 1, index 2, index 3 and IMA levels in the umbilical cord could not be evaluated clearly.
5.1. Conclusions
In our study, we documented an increased antioxidant thiol, natural and total thiol levels compared to disulfide ratios in preterm babies compared to term babies. We believe that evaluation of thiol-disulphide homeostasis in preterm and term infants may be demonstrative for oxidant capacity of the newborn, hence the oxidative stress. Our study also revealed that oxidative stress detected by increasing index 3 increased in preeclamptic mother babies. In our study, the rates of disulfide and index 1 and 2 were lower and index 3 levels were higher in thiol homeostasis in preterm NEC infants. We documented increased index 3 rates in infants with sepsis. All the findings showed us that a small amount of blood to be taken from the umbilical cord after birth will be prevented against the risks that may occur as a result of testing.