1. Introduction
In late 2019, a novel coronavirus named COVID-19 led to a large outbreak in Wuhan, Hubei Province, China. The disease quickly spread to all parts of China and many countries around the world (1, 2). A few cases of pneumonia in newborns and infants with COVID-19 infection have been reported. The neonates described as the cases of COVID-19 were asymptomatic or had mild symptoms. And sampling has been performed for the infants born to mothers with COVID-19 were tested (3, 4). In this report, we describe a case of a male neonate presented with respiratory symptoms that persisted beyond 10 days. The pharyngeal swab test of COVID-19 turned positive using a real-time reverse transcription-polymerase chain reaction (rRT-PCR) method.
2. Case Presentation
A 35-day-old male infant presented to the Emergency Department of Bohlool Hospital, Gonabad, Iran, with respiratory distress and cyanosis. The newborn has been delivered via natural birth at term with birth weight of 3,180 grams. The mother was 32 years old at the time of childbirth. The patient was referred to another center 10 days earlier with the same symptoms and was discharged after five days of hospitalization. In addition, the patient had no history of fever. At the admission to Bohlool Hospital, the patient was suffering from nasal congestion and hypoxemia. The peripheral capillary oxygen saturation (SPO2) was 82%, which increased to 94% following the therapeutic administration of oxygen. The other vital signs included the body temperature of 37.5°C, pulse rate of 140 beats per minute, and respiratory rate (RR) of 65 breaths per minute.
In physical examination, subcostal, intercostal, and suprasternal retractions were observed. In addition, generalized wheezing and mild bilateral crackles were heard especially in the right lung upper zone. There was no history of fever, cough, or myalgia in the family history. By the 11th day of birth, he had passing contact with someone who had been coughing for a short period of time. Laboratory data of the patient on admission and the changes after the beginning of treatment are shown in Table 1.
| Measure | Results (on Admission) | Results (at Discharge Time) |
|---|---|---|
| WBC, × 109/L | 20.1 | 14.3 |
| Neut, × 109/L | 5.02 | 3.8 |
| Lym, × 109/L | 11.65 | 8.2 |
| Hb, g/dL | 11.3 | 11.1 |
| Plt, × 109/L | 686 | 502 |
| ESR, mm/h | 18 | - |
| CRP | Negative | - |
| BUN, mg/dL | 5 | 5.2 |
| Cr, mg/dL | 0.62 | 0.62 |
| Na, mEq/L | 130 | 129 |
| K, mEq/L | 3.9 | 4.1 |
| Calcium, mg/dL | 11.6 | - |
| AST, units/L | 31 | - |
| ALT, units/L | 25 | - |
| ALP, units/L | 276 | - |
| Total bilirubin, µmol/L | 1.1 | - |
| Direct bilirubin, µmol/L | 0.17 | - |
| PT, s | 12.8 | - |
| PTT, s | 37 | - |
| INR | 1.09 | - |
| VBG, primary | ||
| pH | 7.10 | 7.30 |
| pCO2, mmHg | 63 | 45 |
| HCO3, mEq/L | 19.1 | 21.5 |
| VBG (after 1 h) | ||
| pH | 7.24 | |
| pCO2, mmHg | 51 | |
| HCO3, mEq/L | 21.4 | |
| Blood culture | negative | - |
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; Cr, creatinine; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; INR, international normalized ratio; Lym, lymphocyte coun; Neut, neutrophil count; PT, prothrombin time; PTT, partial thromboplastin time; VBG, venous blood gas; WBC, white blood cells.
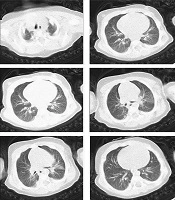
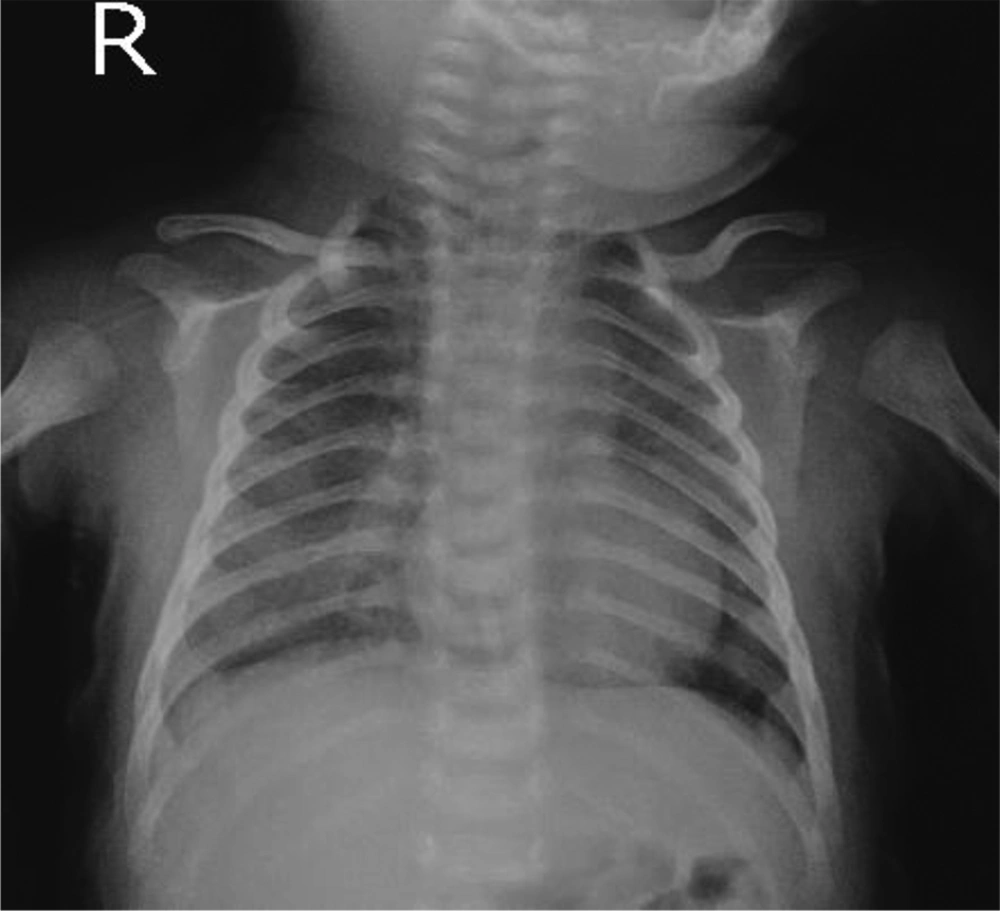
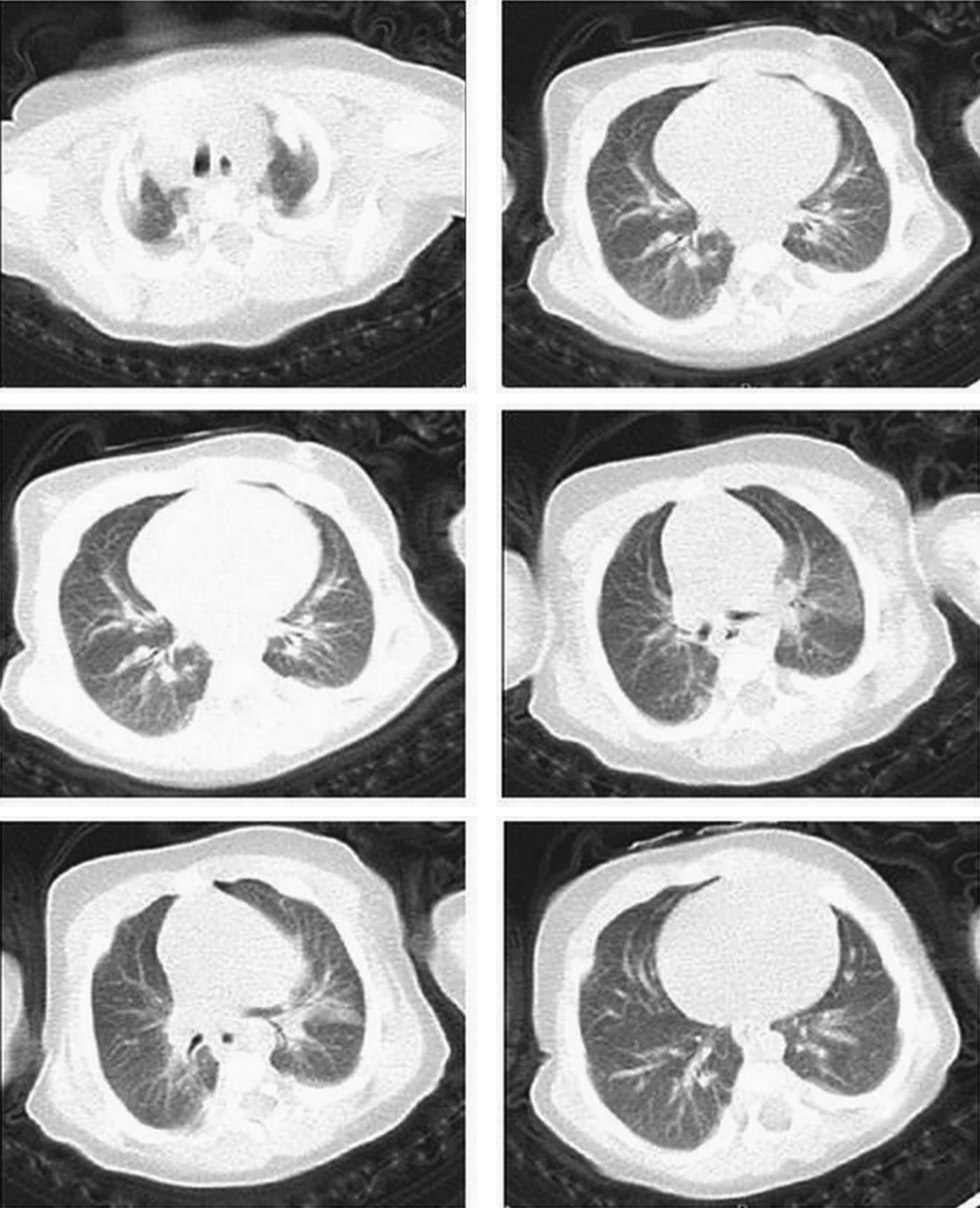
Chest x-ray of the patient showed pulmonary opacity in the right middle lobe, with predominantly peripheral distribution, and haziness in the left upper lobe (Figure 1). These findings were confirmed by CT-scan showing a consolidation in left upper lobe, peripheral patchy ground-glass opacification, and reticulation in right upper, left upper, right middle, and lingual lobes. The mentioned signs were highly suggestive of COVID-19 (Figure 2).
A pharyngeal swab was taken to evaluate COVID-19 infection on the first day of hospitalization. Oxygen was administered using an oxyhood. The patient received two puffs of salbutamol every 20 minutes three times and then every four hours. Simultaneously, azithromycin, vancomycin, meropenem, oseltamivir, and hydroxychloroquine were administered as 10 mg/kg once daily, 15 mg/kg/dose every six hours, 30 mg/kg/dose every eight hours, 12 mg every 12 hours, and 5 mg/kg/day every 12 hours, respectively. Considering the positive results of the rRT-PCR for COVID-19, treatment needed to be continued. On the tenth day of hospitalization rRT-PCR for COVID-19 was negative. Respiratory distress was relieved with the RR of 45 breaths per minute and SPO2 of 98% without oxygen therapy. The patient had complete remission according to clinical findings on the eleventh day of hospitalization and was discharged.
3. Discussion
According to the literature, symptomatic infection with COVID-19 was not common among children, and if present, the symptoms were mild. However, there were several cases reported with severe infection (5-8). Moreover, the symptomatic infection of COVID-19 in newborns was rare and mostly reported following the screening of neonates born to mothers with confirmed COVID-19 (4, 6). In this study, we presented a 35-day-old male infant with respiratory distress persisting beyond 10 days. Considering the time between childbirth and the onset of disease and negative history of COVID-19 in mother, the vertical transmission seemed unlikely.
In China, 10 patients with COVID-19 infection aged between 3 to 131 months were reported. The described symptoms were fever, cough, sore throat, and nasal congestion reported in eight (80%), six (60%), four (40%), and three (30%) patients, respectively. None of the patients had dyspnea during the disease and fever resolved within 24 hours of onset. Four patients (40%) had unilateral patchy infiltration and the lymphocyte count ranged from 1.2 × 106/L to 4.2 × 106/L (9).
Despite previous studies that commonly reported mild infections in children (3, 4, 10), the present study described severe manifestations of the disease including tachypnea, hypoxemia, and pulmonary consolidation. The etiology of milder COVID-19 infection in children has not been fully understood. However, it can partly be related to exposure and host factors. That is, better care at home and less chance of exposure of children with pathogens and sick people plays a role. On the other hand, the less mature Angiotensin-converting enzyme II (ACE2) in children, the higher levels of antiviral antibodies in these patients, the different response of the immune system to the pathogen, given that it is developing, can collectively lead to milder symptoms (10, 11).
Delays to diagnosis and treatment were among the causes of disease severity. Regarding the previous studies, the treatment has been initiated in the early stages of the disease. Therefore, delayed diagnosis of the disease in children and infants may lead to more severe manifestations of COVID-19.
One of the limitations of our study was, because the difficult blood sampling, that we could repeat only the VBG of the patient.
Conclusion
Currently, the outbreak of COVID-19 is affecting many countries around the world. Although rare cases of this disease in infants were reported, the transmission of disease from affected persons to infants can happen. Consequently, family awareness should be raised to protect children. Further studies are recommended to identify clinical and laboratory manifestations of infants with COVID-19 for early diagnosis and management of the disease.