1. Background
Neonatal respiratory distress syndrome (RDS) in new-born infants is a lung disease presenting as respiratory compromise shortly after birth. The etiology of RDS mainly involves developmental immaturity of the lungs (1-4). Intratracheal instillation of pulmonary surfactant is internationally recognized as the most effective treatment for RDS (5) and has saved the lives of many premature infants since its implementation in clinical practice. However, in some infants previously diagnosed with RDS, especially late-preterm and full-term (LP/FT) infants, the effectiveness of treatment has not been satisfactory, despite intensive care and surfactant replacement therapy (SRT).
Many paediatricians have observed that respiratory distress (RD) in newborn infants is only a symptom that can be caused by many respiratory diseases, including RDS, RDS with additional pathogenic factors, and other factors alone (6-9). Based on the definition of neonatal RDS, premature delivery is the most important cause of surfactant deficiency leading to RDS, with lower gestational age (GA) being associated with higher incidence rates; for LP/FT infants, the risk of primary surfactant deficiency should be relatively small. However, due to the lack of standardization of RD diagnosis in China, in actual clinical practice, some of the LP/FT infants with RD but not primary pulmonary surfactant deficiency are also diagnosed with RDS and given exogenous SRT. In many of these LP/FT infants, repeated application of surfactants with conventional doses does not provide significant improvements in lung condition, but the optimal time for treatment may have been missed. Therefore, identification of the causes of RD at different GAs and elucidation of prevention and treatment measures for RD that are based on its cause are urgently needed.
2. Objectives
We designed a study in a large network of neonatal intensive care units (NICUs) in China to compare the therapeutic effects of SRT in infants of different GAs who were previously diagnosed with RDS and further analyse the cause of RD at different GAs according to the differential risk factors closely related to the severity of RDS.
3. Methods
This work was completed by the Chinese Collaborative Study Group for Neonatal Respiratory Diseases. Twenty-six NICUs participated in this survey. These units are major referral centres located in 14 provinces and municipalities and are representative of the health facilities offering newborn intensive care in their respective areas. Participating institutions included 23 general hospitals and 3 children’s hospitals and included both in-born and transported infants. This study was coordinated by Daping Hospital, Army Medical University. The ethics committee of Daping Hospital approved the study protocols according to the Chinese regulations for clinical investigation.
3.1. Participants
The current study involved infants who were diagnosed with RDS and received SRT during hospitalization at 26 hospitals with level 3-4 NICUs in China from 2015 to 2019 (10). According to chest X-ray findings, characteristic clinical features and blood gases, all infants were classified as Grade I, Grade II, Grade III or Grade IV (11).
The infants were supported by different primary modes of ventilation according to RDS severity. Nasal continuous positive airway pressure (NCAP) or nasal intermittent positive pressure ventilation (NIPPV) were provided for grade I and II RDS, and high-frequency oscillatory ventilation (HFOV) or conventional mechanical ventilation (CMV) were provided for grade III and IV RDS. Most infants with RDS received the first dose of surfactant as soon as practicably possible (within 24 h after birth). The primary treatment was a 200 mg/kg dose of porcine surfactant, and an additional 100 mg/kg dose was administered in cases that required repeated treatment.
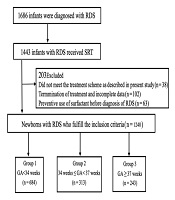
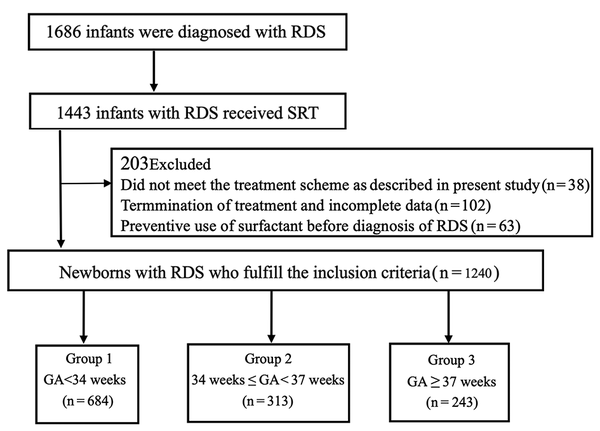
From 2015 to 2019, in these 26 NICUs, 1686 infants were diagnosed with RDS, of whom 1443 (85.6%) received SRT. Thirty-eight patients who did not meet the respiratory support and surfactant administration scheme described above were excluded. An additional 63 patients were excluded because they received a preventive dosage of surfactant before the diagnosis of RDS, and 102 patients were excluded because of termination of treatment and incomplete data. Finally, 1240 patients with complete data were included in the analysis in this multicentre study (Figure 1).
3.2. Study Design
3.2.1. Primary Outcome: Efficacy of SRT in Infants Diagnosed with RDS
The primary outcome was the efficacy of SRT in infants with RDS at different GAs. All subjects recruited for this study were classified into the following three groups: Early-preterm (EP) infant group: GA < 34 weeks; LP infant group: GA 34 0/7 to 36 6/7 weeks; and FT infant group: GA ≥ 37 weeks. The changes in blood gas and oxygenation parameters 12 hours after SRT and the clinical outcomes were used as evaluation indices to compare the therapeutic effects of SRT among infants at the different GAs (according to the surfactant instructions, for the salvage therapy of RDS, 12 hours is the shortest interval before surfactant can be used again if necessary; therefore, most NICUs in China typically re-examine the blood gas, oxygenation index and chest X-ray 12 hours after the first use of surfactant to determine whether surfactant should be used again). New-born infants with incomplete data were not included in this study. To correct for the difference in the RDS severity among the three groups, a subgroup analysis was conducted in infants with RDS grades III and IV to further validate the previous results.
3.2.2. Secondary Outcome: Analysis of Perinatal Risk Factors in Infants Diagnosed with RDS at Different GAs
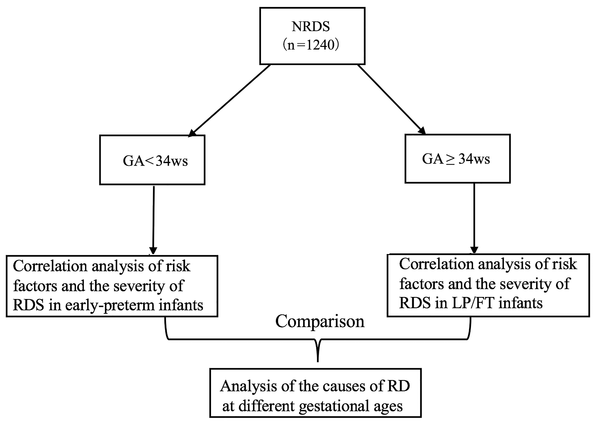
The secondary outcome was the risk factors in infants with RDS at different GAs. All infants were divided into the following two groups: EP infant group: GA < 34 weeks and LP/FT infant group: GA ≥ 34 weeks. In each group, the infants with RDS grades I and II were classified as mild cases, and the infants with RDS grades III and IV were classified as severe cases. Based on previous research (12-17), we listed 30 risk factors that may be related to the onset of RD and associated these risk factors with the severity of RDS at different GAs. By comparing the differences in risk factors closely related to the severity of RDS at different GAs, the cause of RD at different GAs was further analysed (Figure 2 technology roadmap of secondary outcome).
3.3. Statistical Analysis
Regarding the primary outcome, the data were analysed using paired-sample t-tests, one-way ANOVA, or chi-square tests, and the data are expressed as the mean ± standard deviation (SD) or as a percentage.
Regarding the secondary outcome, all risk factors were treated as categorical variables and are reported as frequencies and percentages. We performed forward stepwise variable selection (with an entry criterion of P < 0.05) in a multivariate logistic regression model to investigate the association between the potential risk factors and the severity of RDS at different GAs. All variables with P < 0.20 in the univariate analysis were included in the stepwise variable selection. Receiver operator characteristic curves were used to discriminate risk factors that were closely related to the severity of RDS at different GAs.
P < 0.05 was considered to indicate significant differences. The Statistical Package for the Social Sciences (SPSS) program (16.0 for Windows software, LEAD Technology, Inc.) was used for data analysis.
4. Results
4.1. Patient Characteristics
Among the 1240 infants diagnosed with RDS, 684 were EP infants (55.2%), 313 were LP infants (25.4%), and 243 were FT infants (19.6%). The mean birth weights and 5-min Apgar scores in the LP and FT infant groups were significantly higher than those in the EP infant group (P = 0.000). Similarly, cesarean deliveries were significantly more frequent in the LP and FT infant groups than in the EP infant group (P = 0.000). In addition, the proportion of males in the FT infant group was significantly higher than those in the EP and LP infant groups (P = 0.000) (Table 1). Similar results were observed in the subgroups of infants with RDS of grades III and IV (Table 2).
| GA, wk | P Value | |||
|---|---|---|---|---|
| Early-Paterm Infants (≤ 34) (N = 684) | Near-Term Infants (34 - 37) (N = 313) | Full-Term Infants (≥ 37) (N = 243) | ||
| BW, g | 1.60 ± 0.42 | 2.043 ± 0.49c | 3.05 ± 0.52c | 0.000 |
| Male gender, % | 61.5 | 67.7 | 78.6c, d | 0.000 |
| Caesarean delivery, % | 53.4 | 84.0c | 84.0c | 0.000 |
| 5-min Apgar | 8.62 ± 1.71 | 9.39 ± 1.39c | 9.02 ± 1.68c | 0.000 |
| EV, % | 59.7 | 84.2c | 86.7c | 0.000 |
| MVT, median, h | 89.11 | 91.17 | 94.33 | 0.679 |
| SR, % | 93.5 | 86.96 | 88.13 | 0.537 |
| HT, d | 19.29 ± 20.34 | 15.18 ± 8.16c | 13.75 ± 7.00c | 0.000 |
| RSR, % | 14.31 | 19.13c | 24.78c | 0.001 |
| GA, wk | P Value | |||
|---|---|---|---|---|
| Early-Paterm Infants (≤ 34) (N = 213) | Near-Term Infants (34 - 37) (N = 110) | Full-Term Infants (≥ 37) (N = 136) | ||
| BW, g | 1.52 ± 0.44 | 2.50 ± 0.52c | 3.02 ± 0.49c | 0.000 |
| Male gender, % | 63.8 | 68.2 | 77.9c, d | 0.021 |
| Ceasarean delivery, % | 56.8 | 87.3c | 84.6c | 0.000 |
| 5-min Apgar | 8.06 ± 2.06 | 9.13 ± 1.74c | 8.87 ± 1.84 | 0.000 |
| EV, % | 73.24 | 77.30 | 86.00 | 0.019 |
| MVT, median, h | 96.00 | 81.00c | 84.00c | 0.000 |
| SR, % | 74.60 | 92.70c | 94.10c | 0.000 |
| HT, d | 28.10 ± 24.04 | 15.69 ± 8.04c | 14.10 ± 6.02c | 0.000 |
| RSR, % | 29.60 | 33.60 | 34.60 | 0.572 |
4.2. Primary Outcome: Efficacy of SRT in Infants with RDS
4.2.1. Total Data Analysis (N = 1240)
Among all infants receiving SRT for RDS, the mean age on receiving the first dose of surfactant was 3.8 hours after birth (range: 0.2 - 16.5 hours), and no significant difference was observed among the three groups (P < 0.05).
Blood gas analysis was performed before and after the first dose of surfactant was administered. Twelve hours after SRT, a significant increase in pH was observed in the EP and LP infant groups (P = 0.000), while no significant difference in pH before and after the surfactant administration was observed in the FT infant group (P = 0.060). After therapy, the PaO2 level was significantly increased in the EP infant group (P = 0.000), but no significant differences in this value were observed in the LP and FT infant groups (P = 0.209, P = 0.761). Furthermore, 12 hours after the surfactant administration, the PaCO2 level in the EP infant group was significantly decreased (P = 0.000). While no significant differences in the PaCO2 level were observed in the LP and FT infant groups (P = 0.233, P = 0.103), an increasing trend in the PaCO2 level was observed in the FT infant group (Table 3).
| Group | pH | P Value | PaO2 | P Value | PaCO2 | P Value | FiO2 | P Value | P/F | P Value | PaO2/PAO2 | P Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before Treatment | 12 h After Treatment | Before Treatment | 12 h After Treatment | Before Treatment | 12 h After Treatment | Before Treatment | 12 h After Treatment | Before Treatment | 12 h After Treatment | Before Treatment | 12 h After Treatment | |||||||
| Early-preterm infants (n = 684) | 7.24 ± 0.10 | 7.35 ± 0.09 | 0.000 | 70.57 ± 33.48 | 86.04 ± 33.01 | 0.000 | 53.73 ± 12.68 | 39.69 ± 10.53 | 0.000 | 0.40 ± 0.13 | 0.38 ± 0.18 | 0.002 | 193.53 ± 110.87 | 261.34 ± 130.25 | 0.000 | 0.39 ± 0.41 | 0.48 ± 0.25 | 0.000 |
| Near-term infants (n = 313) | 7.24 ± 0.09 | 7.33 ± 0.10 | 0.000 | 74.52 ± 25.06 | 76.93 ± 26.27 | 0.209 | 48.60 ± 9.81 | 47.56 ± 14.30 | 0.233 | 0.39 ± 0.13 | 0.40 ± 0.17 | 0.419 | 210.85 ± 99.78 | 223.88 ± 113.07 | 0.061 | 0.40 ± 0.22 | 0.43 ± 0.24 | 0.077 |
| Full-term infants (n= 243) | 7.25 ± 0.10 | 7.27 ± 0.13 | 0.060 | 72.82 ± 21.71 | 72.33 ± 20.67 | 0.761 | 49.16 ± 12.16 | 50.84 ± 13.67 | 0.103 | 0.41 ± 0.14 | 0.44 ± 0.19 | 0.017 | 201.11 ± 101.17 | 201.87 ± 110.05 | 0.920 | 0.39 ± 0.32 | 0.40 ± 0.29 | 0.566 |
| P value | 0.075 | 0.000 | 0.062 | 0.000 | 0.000 | 0.000 | 0.201 | 0.001 | 0.056 | 0.000 | 0.871 | 0.000 | ||||||
Oxygenation function parameters were analysed before and after the first dose of surfactant was administered. Twelve hours after SRT, the FiO2 level was significantly decreased in the EP infant group (P = 0.002) but was not significantly altered in the LP infant group (P = 0.419) and was significantly increased in the FT infant group (P = 0.017). After therapy, the PaO2/FiO2 (P/F) ratio was significantly increased in the EP infant group (P = 0.000) but was not significantly different in the LP and FT infant groups (P = 0.061, P = 0.920). Similarly, the PaO2/PAO2 ratio was significantly improved in the EP infant group (P = 0.000) but was not significantly different in the LP and FT infant groups (P = 0.077, P = 0.566) (Table 3).
4.3. Therapeutic Outcomes
The rates of tracheal intubation (cases where surfactant was administered through the tracheal intubation tube and the patient was extubated soon after the surfactant was administered were not included in the intubation rate statistics) and repeated surfactant administration in the LP and FT infant groups were significantly higher than those in the EP infant group (P = 0.000, P = 0.001). Although no significant differences were observed in the mechanical ventilation time or survival rate, trends toward an increased mechanical ventilation time and a decreased survival rate were observed with increasing GA. Due to the low GAs, immature development of multiple organs, and numerous complications in EP infants, their hospitalization times were significantly longer than those of infants in the LP and FT infant groups (P = 0.000) (Table 1).
4.4. Subgroup Analyses (RDS Grades III and IV, N = 497)
In the subgroup of infants with RDS of grades III and IV, the changes in blood gas and oxygenation function parameters before and after the first dose of SRT were similar to those in infants with all RDS grades. Twelve hours after the SRT, significant improvements in blood gas results and oxygenation function parameters were observed in the EP infant group (P < 0.05), but no significant differences were observed in the LP and FT infant groups (P > 0.05) (Table 4).
| Group | pH | P Value | PaO2 | P Value | PaCO2 | P Value | FiO2 | P Value | P/F | P Value | PaO2/PAO2 | P Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before Treatment | 12 h After Treatment | Before Treatment | 12 h After Treatment | Before Treatment | 12 h After Treatment | Before Treatment | 12 h After Treatment | Before Treatment | 12 h After Treatment | Before Treatment | 12 h After Treatment | |||||||
| Early-preterm infants (n = 213) | 7.23 ± 0.10 | 7.34 ± 0.11 | 0.000 | 68.26 ± 33.50 | 84.65 ± 33.39 | 0.000 | 52.39 ± 12.05 | 41.09 ± 10.87 | 0.000 | 0.42 ± 0.11 | 0.39 ± 0.16 | 0.001 | 8.94 ± 4.87 | 5.49 ± 3.65 | 0.000 | 0.32 ± 0.18 | 0.47 ± 0.27 | 0.000 |
| Near-term infants (n = 110) | 7.25 ± 0.11 | 7.28 ± 0.15 | 0.066 | 69.52 ± 25.53 | 74.66 ± 17.85 | 0.071 | 49.93 ± 10.51 | 47.34 ± 13.42 | 0.099 | 0.40 ± 0.15 | 0.40 ± 0.20 | 0.948 | 8.08 ± 5.65 | 6.88 ± 5.58 | 0.075 | 0.39 ± 0.26 | 0.45 ± 0.24 | 0.027 |
| Full-term infants (n = 136) | 7.24 ± 0.11 | 7.27 ± 0.15 | 0.062 | 67.83 ± 22.33 | 70.95 ± 18.03 | 0.092 | 49.60 ± 12.97 | 49.81 ± 13.46 | 0.886 | 0.41 ± 0.15 | 0.44 ± 0.20 | 0.107 | 7.69 ± 5.18 | 8.17 ± 5.62 | 0.308 | 0.38 ± 0.40 | 0.38 ± 0.25 | 0.942 |
| P value | 0.356 | 0.000 | 0.893 | 0.000 | 0.062 | 0.000 | 0.198 | 0.037 | 0.071 | 0.000 | 0.060 | 0.012 | ||||||
Because the GA of most infants with severe RDS in the EP infant group was less than 30 weeks, these infants were characterized as having multiple immature organs and numerous complications. Therefore, the mechanical ventilation times and hospitalization times of these infants were significantly longer than those in the LP and FT infant groups (P = 0.000, P = 0.000), and the survival rate in the EP infant group was also significantly lower than that in the LP and FT infant groups (P = 0.000). However, the rates of tracheal intubation in the LP and FT infant groups remained significantly higher than that in the EP infant group (P = 0.019). No significant difference in the rate of repeated SRT was observed among the three groups (P = 0.572), but a trend toward an increasing rate of repeated SRT was observed with increasing GAs (Table 2).
4.5. Secondary Outcome: Analysis of Perinatal Risk Factors for RDS at Different GAs
Among the baseline characteristics: a GA < 30 weeks, a 5-minute Apgar score < 7, and a lack of prenatal corticosteroid use were risk factors for the severity of RDS in EP infants less than 34 weeks of GA. In the LP/FT infant groups, GAs ranging from 37 to 39 weeks and a 5-minute Apgar score < 7 were also risk factors for the severity of RDS; however, the odds ratios (ORs) of these risk factors in the LP/FT infants were lower than those in the EP infants. In addition, cesarean section was a risk factor for severe RDS in the LP/FT infant group but was not associated with the severity of RDS in the EP infant group (Table 5).
| Risk Factors | RDS < 34 Weeks | RDS ≥ 34 Weeks | |||||
|---|---|---|---|---|---|---|---|
| Cases | Odds Ratio [95% CI] | P Valuea | Cases | Odds Ratio [95% CI] | P Valuea | ||
| Baseline Characteristic | |||||||
| GA | |||||||
| < 30 weeks | 159 | 31.12 [19.13 - 50.61] | 0.000 | ≥ 34 weeks, < 37 weeks | 160 | Reference | |
| ≥ 30 weeks, < 34 weeks | 89 | ≥ 37 weeks, < 39 weeks | 60 | 2.08 [1.41 - 3.06] | 0.003 | ||
| ≥ 39 weeks | 29 | 1.63 [0.97 - 2.76] | 0.178 | ||||
| 5-min Apgar < 7 | 237 | 142.65 [73.39 - 277.26] | 0.000 | 35 | 2.30 [1.29 - 4.09] | 0.012 | |
| Caesarean delivery | 137 | 0.531 [0.39 - 0.73] | 0.000 | 27 | 2.31 [1.42 - 3.75] | 0.002 | |
| Lack of AS | 101 | 1.54 [1.12 - 2.10] | 0.025 | 29 | 1.26 [0.74 - 2.16] | 0.613 | |
| Han nationality | 213 | 1.03 [0.66 - 1.61] | 0.998 | 245 | 0.41 [0.07 - 2.26] | 0.557 | |
| Male | 153 | 0.98 [0.71 - 1.35] | 0.998 | 183 | 1.18 [0.81 - 1.72] | 0.613 | |
| Twins | 67 | 1.39 [0.97 - 2.00] | 0.172 | 29 | 1.24 [0.72 - 2.13] | 0.657 | |
| BW | |||||||
| < 10% | 23 | 0.61 [0.34 - 1.09] | 0.208 | 28 | 0.95 [0.6 - 1.2] | 1 | |
| ≥ 90% | 8 | 0.88 [0.36 - 2.14] | 1 | 18 | 0.72 [0.36 - 1.44] | 0.532 | |
| ≥ 10%, < 90% | 217 | Reference | 203 | Reference | |||
| Perinatal Infection | |||||||
| Chorioamnionitis | 91 | 1.98 [1.41 - 2.79] | 0.000 | 93 | |||
| Early septicaemia | 110 | 2.91 [2.08 - 4.08] | 0.000 | 119 | |||
| AFC | 200 | 0.89 [0.80 - 0.99] | 0.079 | 181 | |||
| IDOM | 84 | 1.19 [0.85 - 1.66] | 0.556 | 87 | |||
| PMR | 69 | 1.45 [1.01 - 2.07] | 0.125 | 47 | |||
| Perinatal Hypoxia | |||||||
| PA | 29 | 4.75 [2.37 - 9.48] | 0.000 | 20 | |||
| Oligohydramnios | 16 | 4.29 [1.74 - 10.57] | 0.007 | 29 | |||
| FID | 63 | 1.02 [0.71 - 1.45] | 0.998 | 186 | |||
| DPP | 14 | 0.96 [0.48 - 1.87] | 0.998 | 50 | |||
| UCA | 27 | 1.00 [0.61 - 1.65] | 0.998 | 147 | |||
| Mother-Associated Risk Factors | |||||||
| MA, y | |||||||
| < 19 | 5 | 0.22 [0.04 - 1.15] | 0.208 | 2 | |||
| 20 - 35 | 225 | Reference | 218 | Reference | |||
| > 35 | 18 | 1.05 [0.58 - 1.90] | 1 | 29 | |||
| PIH | 31 | 0.54 [0.35 - 0.83] | 0.079 | 30 | |||
| GDM | 23 | 0.63 [0.37 - 1.04] | 0.172 | 23 | |||
| Anaemia | 6 | 0.97 [0.35 - 2.66] | 0.998 | 7 | |||
| Hypothyroidism | 6 | 0.89 [0.33 - 2.40] | 0.998 | 29 | |||
| Hyperthyroidism | 3 | 1.34 [0.30 - 6.04] | 0.998 | 7 | |||
| ICP | 1 | 0.22 [0.03 - 1.77] | 0.319 | 7 | |||
| GT | 2 | 0.59 [0.12 - 2.95] | 0.808 | 2 | |||
| PWHD | 4 | 7.23 [0.80 - 65.05] | 0.172 | 0 | |||
| PCWISD | 0 | 0.00 [0.00 - Inf] | 0.998 | 3 | |||
Risk Factors for Severe RDS by Multivariate Logistic-Regression Analysis
Among the risk factors related to perinatal infection: chorioamnionitis, early septicaemia, amniotic fluid contamination, infectious disease of the mother, and premature membrane rupture were risk factors for RDS severity in the LP/FT infant groups. Although chorioamnionitis and early septicaemia were also risk factors for RDS severity in the EP infant group, the ORs of these risk factors were lower than those in the LP/FT infant groups (Table 5).
Among the risk factors related to perinatal hypoxia: oligohydramnios, fetal intrauterine distress, dangerous placenta praevia, and umbilical cord abnormalities were closely related to the severity of RDS in the LP/FT infant groups. Only placental abruption and oligohydramnios were risk factors for severe RDS in the EP infant group, and the OR of oligohydramnios in the EP infant group was lower than the ORs in the LP/FT infant groups (Table 5).
Among the risk factors associated with maternal complications during pregnancy: hypothyroidism was the only risk factor related to the severity of RDS in the LP/FT groups, and no risk factor was found to be related to the severity of RDS in the EP infant group (Table 5).
5. Discussion
The current report presents analyses of new-born infants who were diagnosed with RDS and received SRT at 26 NICUs from 2015 to 2019, covering half of the provinces in China.This study included a substantial number of subprovincial tertiary centres, with the number of patients enrolled totaling several times the number in a previous survey (18).
In the present survey, we can conclude that compared with those of LP and FT infants with RDS, significantly better blood gas conditions and oxygenation function parameters and a lower rate of endotracheal ventilation were associated with SRT in EP infants with RDS. Most EP infants received surfactant only once, while more infants required multiple doses of surfactant in the LP and FT infant groups. These results suggest that SRT for RDS was more effective in the EP infants than in the LP and FT infants. Although we excluded infants with TTN, the cesarean rates were still very high in the LP/FT infant groups with RDS, suggesting that cesarean may be associated with other mechanisms leading to severe respiratory distress in addition to delaying lung fluid absorption. These findings were consistent with the results of our previous single-centre study (18) and with data in the literature (19, 20), supporting the finding that the pathogenic mechanism of RD in LP and FT infants differs from that in EP infants < 34 weeks of GA, some of the LP and FT infants with RD symptoms might be misdiagnosed as RDS.
Based on clinical epidemiology research, to further explore the etiology and pathogenesis of RD at different GAs, we listed 30 perinatal risk factors (see Figure 2 for details) (21-25) that may be closely related to RD, and the relationships between these 30 risk factors and the severity of RDS at different GAs were analysed. The current results showed that the perinatal risk factors associated with severe RDS varied among infants of different GAs; the severity of RDS in EP infants may be associated with a lower GA, immature lung development, and lack of surfactant secretion. By contrast, the severity of RDS in LP/FT infants is more likely related to perinatal infection, hypoxia, and cesarean delivery. These results further indicate that the etiology of RD in LP and FT infants may differ from that in EP infants < 34 weeks’ GA, and thus some LP and FT infants with RD symptoms should not be diagnosed with RDS.
The following questions need to be answered by pediatricians: How do perinatal infection and perinatal hypoxia lead to RD in infants? In addition to TTN caused by delayed lung clearance, is cesarean section associated with other mechanisms that lead to severe RD? Do any differences exist between these types of RD and traditional RDS caused by primary surfactant deficiency? Do any particularities exist in the formulation of a treatment program?
After obtaining consent from the parents, we performed autopsies on eight of the LP/FT infants who died of RD. The pathological sections of lung tissues from the infants showed that the alveoli were obviously dilated due to lung fluid, which clearly differed from the widespread collapse of the alveoli caused by the lack of surfactant in the EP infants (unpublished data). Anderson et al also confirmed that delayed pulmonary fluid absorption is an important cause of RD in animal experiments (26). In their experiments, the mice died within 40 hours of knocking out the key gene in alpha epithelial sodium channels (α-ENaC), which affected the clearance of lung fluid. Altogether, these data and clinical findings suggest that pulmonary edema may play an important role in the onset of RD in LP and FT infants. However, this type of RD in LP/FT infants was not defined until August 2017, when “neonatal acute respiratory distress syndrome (ARDS)” was defined for the first time by the European Society for Paediatric and Neonatal Intensive Care (ESPNIC) and the European Society for Paediatric Research (ESPR). This definition is known as the Montreux definition of neonatal ARDS (27). Pulmonary edema is an important pathological feature of neonatal ARDS.
Regarding possible pathogenic factors underlying neonatal pulmonary edema, we previously found that a-ENaC plays an important role in the pathogenesis of respiratory distress by influencing the activity of pulmonary surfactant and lung liquid absorption in neonates, and the SCNN1A gene that encodes a-ENaC might be an important gene that predisposes neonates to RD (28). For infants suffering from perinatal infection and hypoxia, we hypothesize that after exposure to perinatal infection and chronic intrauterine anoxia caused by various factors, alveolar II epithelial cells become damaged, thus increasing the permeability of alveolar epithelial cells and vascular endothelial cells, which leads to inflammatory exudation and pulmonary fluid excretion disorder and ultimately to pulmonary edema and secondary surfactant deficiency or decreased surfactant activity; these processes eventually lead to ARDS (29). Regarding the effects of cesarean delivery on severe RD caused by neonatal pulmonary edema, whether it is an independent risk factor or combined with other risk factors remains to be further explored. These types of RD can occur in new-borns of all GAs, have often been observed in LP and FT infants, and are considered refractory RD (30).
Influenced by work intensity and the modern maternal reproductive age, the elective cesarean rate as well as perinatal infection- and perinatal hypoxia-associated risk factors have significantly increased in recent years in China. The mortality of RD associated with these etiologies is significantly higher than that of RDS caused by primary surfactant deficiency in EP infants. Therefore, to clarify the specific pathogenesis of these types of RD, an early diagnosis of RDS, ARDS or other types of RD, which is important for the timely and effective application of comprehensive treatment measures, has become a new challenge for pediatricians. In addition, because of the lack of sufficient health insurance for newborns and the high price of surfactant in China, some parents cannot afford the high cost of treatment and choose to terminate treatment. If the cause of RD can be judged correctly and sufficiently early, not only can the mortality of infants with RD be reduced, but the blind use of surfactant can often be avoided, reducing the financial burden on parents.
One of the limitations of this study is the lack of a control group of healthy newborns for comparison with RDS infants in the analysis of secondary outcomes. Since this study was a retrospective study, it was difficult to obtain data of healthy newborns at the same time, and so we used different severities of RDS for comparison. Moreover, while this was a large retrospective cohort study, the results are still limited to China. Therefore, due to differences in neonatal/perinatal care within China and between China and other countries, differences in genetic backgrounds, and differences in NICU settings and environmental conditions, these data may not be directly applicable to the rest of the world.
5.1. Conclusions
The current survey revealed that SRT was less effective in LP and FT infants than in EP infants. Most RD in LP and FT infants is more likely related to severe pulmonary edema caused by perinatal infection, perinatal hypoxia and cesarean section, and infants with these risk factors may experience more severe RD and higher mortality; some of these cases may belong to the category of neonatal ARDS. Further research on the roles of perinatal infection- and hypoxia-associated risk factors, elective caesarean delivery and hereditary factors in the pathogenesis of neonatal RD is required to confirm our findings. New strategies for the treatment of refractory RD should concentrate more on pulmonary edema and neonatal ARDS.