1. Context
Children with cerebral palsy may present with voiding dysfunction which usually manifests as urinary incontinence, besides abnormalities of gross motor function and posture (1). Some studies attribute their susceptibility for urinary tract infection (UTI) to voiding dysfunction caused by upper motor neuron lesion and neurogenic bladder (2, 3). Others link the voiding dysfunction to detrusor-sphincter dyssynergia (4, 5). As a common infectious cause of morbidity in childhood (6), UTI may, in the future, lead to renal scarring, and subsequently to hypertension and end-stage kidney disease if it is not appropriately and promptly treated (7-9).
Although the management protocol of cerebral palsy involves a multi-disciplinary approach, routine screening and treatment for UTI may become an important component if the risk and prevalence of the infection in these children are high. For instance, the evidence from published systematic reviews and meta-analyses confirm that the pooled UTI prevalence rate in severe acute malnutrition (SAM) is significantly high (10, 11). To improve disease outcomes in affected children, some authors thus recommend the incorporation of screening and treatment for UTI in their management protocol (10).
Some researchers who investigated UTI prevalence in pediatric patients with cerebral palsy reported relatively low rates (12-14). One of the studies even suggested that these patients are not more susceptible to UTI than their normal and healthy counterparts (12). However, other studies reported very high UTI prevalence rates in children and adolescents with this neurologic disorder (5, 15-17). These disparities may be due to the non-uniformity of study designs and the possibility of different forms of bias in these studies. More importantly, the definition of UTI adopted by the investigators may also have contributed to the absence of unanimity in the findings.
To justify the recommendation for routine screening and early treatment for UTI in children and adolescents with cerebral palsy, the level of evidence of any related research should be adjudged high. Thus, we initiated a systematic review to determine the risk and pooled prevalence rate of UTI in these patients. We also investigated the bacterial isolates and their antibiotic-sensitivity patterns. The review was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.
2. Evidence Acquisition
2.1. Search Strategy
We searched electronic databases -comprising PubMed and Google Scholar- for articles published between 1980 and 2020. (Date of the last search: 4 April 2020). We searched both databases using these descriptors (alone and in combination): ‘urinary tract infection’, ‘bacteriuria’, ‘pyuria’, ‘cerebral palsy’, ‘prevalence’, ‘risk’, ‘children and adolescents’.
2.2. Eligibility and Exclusion Criteria
Eligible primary studies included in this review met the following criteria: (i). observational studies of children without racial, socioeconomic, and educational bias (ii). Full-text studies published in English language (iii). Studies that reported an association between cerebral palsy and UTI or UTI prevalence in affected children and healthy comparators, with a clear definition of these co-morbidities. Excluded articles were abstracts, letters to the Editor, reviews, commentaries, editorials, and studies without primary data. Definition of UTI in selected studies should conform to its standard definition as the presence of pyuria and significant bacteriuria or bacterial colony-forming units (CFU)/mL corresponding to the sampling method of the urine specimen (e.g. 103 CFU/mL for a urine specimen from suprapubic aspiration, > 104 CFU/mL for a catheter specimen and > 105 CFU for midstream specimen) (18). We also included studies which classified cerebral palsy according to the gross motor function classification system (GMFCS); a system that graded the functional impairment of children with this disorder, focusing on self-initiation of the gross motor functions of sitting and walking (19).
2.3. Study Selection
We independently screened the abstracts of retrieved published articles. We obtained and further assessed potentially eligible full-text articles for final inclusion to the list of articles to be reviewed. We removed all duplicates during the study selection process and resolved disagreements until we reached a consensus on selecting an eligible study.
2.4. Quality Assessment
We assessed the quality of included studies with the Newcastle-Ottawa Scale (NOS) (20). The NOS assesses case-control and cross-sectional studies using criteria grouped into ‘selection’ (maximum of 5 stars), ‘comparability’ (maximum of 2 stars), and ‘exposure/outcome’ (maximum of 3 stars). We categorized the star-rating as low if < 7 stars or high if > 7 stars. We independently assessed the quality of these studies and resolved inter-rater discrepancies by consensus.
2.5. Data Extraction and Data Items
We independently retrieved relevant data from the selected studies with a preconceived data-extraction form, designed to obtain information about the first author’s name, year of publication, year of study, study setting and country, study design, study population, sample size and participants’ age and sex distribution. We extracted information on urine sampling and testing methods, diagnostic criteria for UTI and cerebral palsy, and the proportion of study participants with UTI, the participants’ subgroup differences in proportions, as well as the bacterial isolates and their antibiotic sensitivity patterns. Inter-rater reliability for qualitative items was measured using Cohen’s kappa coefficient (κ) (21).
2.6. Risk of Bias in Individual Studies
We explored the risk of bias in each study, with emphasis on sampling bias (which could affect the external validity of study results), data bias, possible attrition bias, and exclusion bias.
2.7. Summary Measure
We analyzed the aggregate data on UTI prevalence in children and adolescents with cerebral palsy, using the log odds ratio (OR) at 95% confidence interval as the summary estimate. Where OR was not reported in any of the case-control studies, we estimated and compared these values in a forest plot.
2.8. Risk of Bias Across Studies
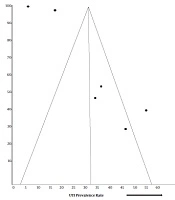
We assessed publication bias across the included studies by displaying the studies for visualization in a funnel plot.
3. Results
3.1. Study Selection
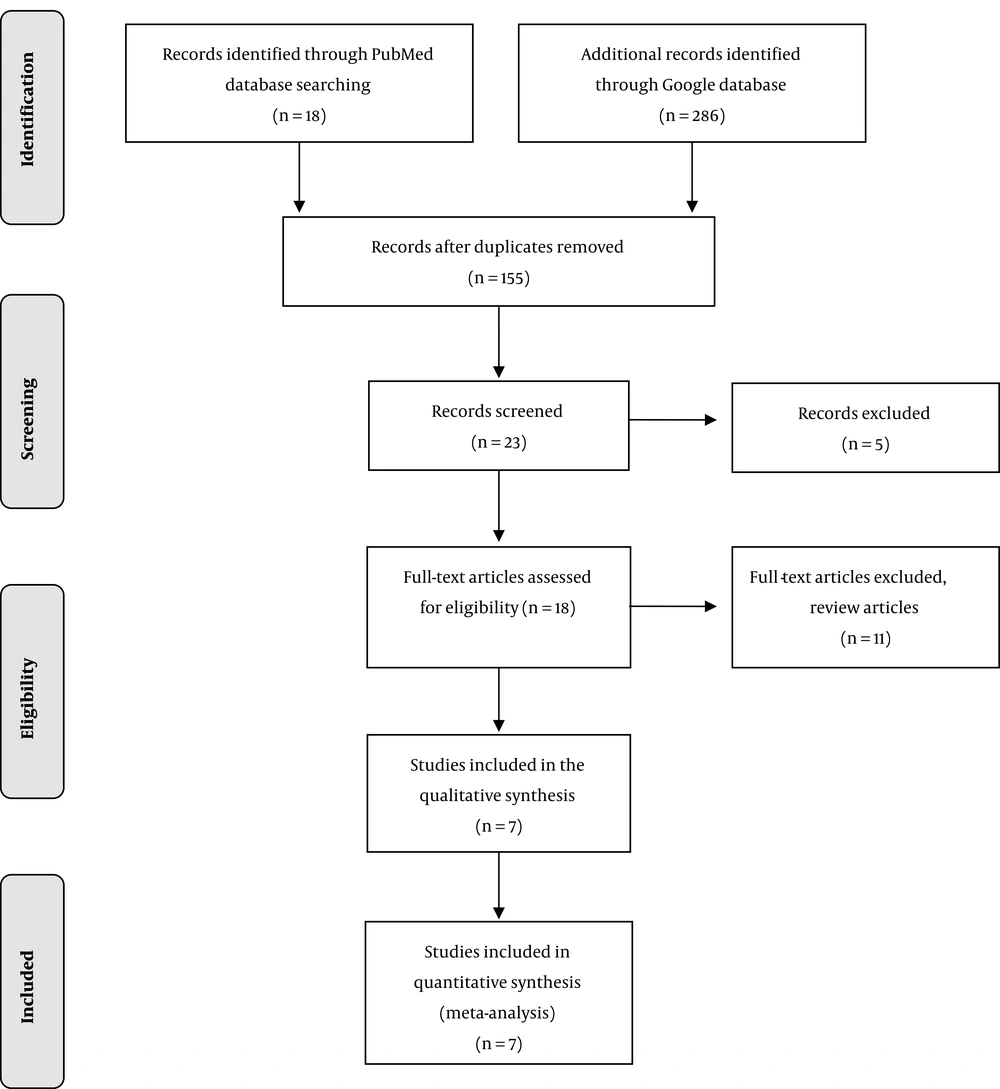
We identified 18 records in the PubMed database and 286 records in Google database: giving a total of 304 records. After removing duplicates, the number of records was scaled down to 155. Only 23 records were left after an initial screening. Five records were further excluded leaving behind 18 full-text original articles, which were assessed for eligibility based on the inclusion criteria. Eleven articles, including review articles, were finally excluded from the list to give only seven original articles for the present review (Figure 1). For these selected studies, Cohen’s κ for inter-rater reliability was calculated to be 0.86.
3.2. Study Characteristics
Of the seven reviewed studies, only two (28.6%) were case-control studies (15, 16); while five (71.4%) were cross-sectional studies (5, 12-14, 17). Two cross-sectional studies were retrospective (5, 17). Additionally, all the studies were hospital-based and were conducted in developed countries like the United Kingdom (5), United States (12), Turkey (16), and developing countries like Brazil (17), Iran (13), Nigeria (15) and Tanzania (14). A population of > 50 subjects was noted in four studies (57.1%), while < 50 subjects were reported in three studies (42.9%). The largest sample size was 100 subjects (12), whereas the least was 27 subjects (5). Subjects’ age and sex distribution were reported in six studies (5, 12-14, 17), but absent in one study (12). Subjects’ age range in all the six studies varied from four months to 20 years. Female predominance was noted in three studies (5, 13, 17), while male subjects predominated in three studies (14-16). The two case-control studies (15, 16) were rated high (> 7 stars) while the cross-sectional studies (5, 12-14, 17) were rated low (< 7 stars) (Table 1).
| Source (First Author’s Name and Year of Publication) | Country of Study | Study Setting and Period of Study | Study Population (Sample Size and Age/Sex Distribution) | Study Design | Study Qualitya |
|---|---|---|---|---|---|
| Hellquist et al. 1985 (12) | United States | Children’s Rehabilitative Hospital, Duke University Medical Center, Durham, North Carolina (Period of study not stated) | 100 children with cerebral palsy (Information about age/sex distribution not available) | A prospective cross-sectional study | 5 stars (low) |
| Reid et al. 1993 (5) | United Kingdom | Pediatric Neurourology Clinic, Guy’s Hospital London (10 years) | 27 children with cerebral palsy (age range: 320 years, mean age = 9.9 years, males = 9, females = 18) | A retrospective, crosssectional descriptive study | 6 stars (low) |
| Ozturk et al. 2006 (16) | Turkey | Isparta Spastic Children’s Center & Suleyman Demirel University Hospital (March to April 2002) | 45 children with cerebral palsy (age range:2-16 years, mean age = 8.4 ± 4.1 years, males = 27, females = 18); 37 siblings of children with cerebral palsy as the control group (mean age = 8.8 ± 4.1 years, males = 21, females = 16); 37 healthy children as control group (mean age = 8.2 ± 3.7 years, males = 21, females = 16) | A prospective, case-control study | 7 stars (high) |
| Silva et al. 2009 (17) | Brazil | SARAH Network of Rehabilitation Hospitals in Rio de Janeiro (January 2003 to June 2005) | 37 children and adolescents with cerebral palsy and lower urinary tract symptoms (age range: 1 - 17 years, mean age = 7.8 ± 4.6 years, males = 16, females = 21) | A retrospective cross-sectional study | 5 stars (low) |
| Anígilájé et al. 2013 (15) | Nigeria | Neurologic Clinic of the Paediatrics Outpatient Department of the Federal Medical Centre, Makurdi. (December 2011 to May 2013) | 52 children with cerebral palsy (age range: 2 - 15 years, mean age = 8.63 ± 3.83 years, males = 30, females = 22) | A prospective, case-control study | 8 stars (high) |
| Fahimzad et al. 2013 (13) | Iran | Mofid Children’s Hospital, Tehran (September 2006 to September 2007) | 60 children with disability; 11 with cerebral palsy (age range: 4 - 168 months, mean age = 53 months, males = 25, females = 35) | A prospective cross-sectional study | 4 stars (low) |
| Ryakitimbo et al. 2018 (14) | Tanzania | Kilimanjaro Christian Medical Centre Neurological Pediatrics Outpatient Clinic, Moshi. (September 2016 to March 2017) | 99 children with cerebral palsy (age range: 2 - 18 years, median age of 4 years, IQR = 3 - 8 years, males = 58, females = 41) | A prospective analytical, cross-sectional study | 6 stars (low) |
aStudy quality was assessed using the star rating of the Newcastle-Ottawa Scale (NOS).
The case-control studies (15, 16) and two cross-sectional studies (12, 14) primarily investigated UTI prevalence in children and adolescents with cerebral palsy. The remaining cross-sectional studies investigated lower urinary tract dysfunction in affected children and adolescents (5, 17) and common infectious indications for hospital admission in children with disabilities, including cerebral palsy (13). Subgrouping of subjects based on major disease types (pyramidal, extrapyramidal and mixed) and grades (GMFCS I-V) was reported in four studies (5, 14-16): with one study (15) also grouping the subjects based on their intellectual ability using the Ziler’s Man-Drawing (ZMD) Quotient (Table 2). Only three studies indicated the urine sampling methods (14-16); urine specimen was collected by the clean-catch or midstream method in two studies (15, 16), and with the urethral catheter in one study (14). Urine testing was conducted in four studies: screening and repeat urine cultures (Uricult®) (12), dipstick urinalysis and urine culture on blood and MacConkey agar (16), dipstick urinalysis, urine microscopy for significant microscopic pyuria and urine culture on sheep blood, MacConkey and cysteine lactose electrolyte deficient (CLED) agar (15), as well as dipstick urinalysis and urine culture on MacConkey and blood agar (14). There was uniformity in defining UTI (as > 105 colony forming units (CFU) per mL urine of a single uropathogen) in two studies whose urine collection methods were reported as midstream or clean-catch (15, 16). However, other studies used different definitions. One study (12), in which the urine sampling method was not stated, defined UTI as > 104 colonies per mL. Perhaps, the authors investigated UTI with catheter urine specimens, which may justify this definition. For the study that reported urethral catheterization (14), UTI was defined as the growth of a single uropathogen with at least 5 × 104 CFU per microliter. Again, the variation in definition could be explained by the authors’ adoption of the revised American Academy of Pediatrics (AAP) guideline for UTI (22). Nevertheless, three studies defined UTI either based on symptoms alone (5) or based on the combination of UTI symptoms and a significant urine culture (12, 16). Studies that defined UTI based on colony counts of > 105 and > 104 apparently adopted the United Kingdom’s National Institute of Health and Clinical Excellence (NICE) guideline for UTI (23).
| Source | Classification of Cerebral Palsy | Grades and Types of Cerebral Palsya | Urine Sampling Method | Methods of Urine Testing | Definition of UTI |
|---|---|---|---|---|---|
| Hellquist et al. 1985 (12) | -Not available | -Not stated | -Not stated | -Screening and repeat urine cultures (Uricult®) | -> 104 colonies per ml |
| -UTI symptoms | |||||
| Reid et al. 1993 (5) | -Not available | -Spastic diplegia | -Not stated | -Not stated | -UTI symptoms |
| -Spastic quadriplegia | |||||
| -Spastic hemiplegia | |||||
| -Mixedb (diplegia and quadriplegia) | |||||
| -Spastic monoplegia | |||||
| Ozturk et al. 2006 (16) | -Based on GMFCS | -Spastic diplegia | -Clean-catch or midstream urine sample collected in a plastic urine collection bag | -Urinalysis strip testing for nitrite, blood, leukocyte esterase, and pH | ->105 colony-forming units per ml urine of a single micro-organism |
| -Spastic quadriplegia | |||||
| -Athetoid | |||||
| -Hypotonic | -A urine culture on blood and MacConkey agars incubated at 37°C | -UTI symptoms | |||
| -Mixed | |||||
| -GMFCS I-II (mild) | |||||
| -GMFCS III-V (severe) | |||||
| Silva et al. 2009 (17) | -Not available | -Not stated | -Not stated | -Urine culture | -Not stated |
| Anigilaje et al. 2013 (15) | -Based on GMFCS and ZMD Quotient | -Spastic hemiplegia | -Mid-stream urine sample | -Urinalysis strip testing for proteinuria, significant hematuria, nitrite and pyuria | -Quantitative growth of bacteria > 105 colony forming units per ml urine, of the same organism (without UTI symptoms)c |
| -Spastic diplegia | |||||
| -Spastic quadriplegia | |||||
| -Mixedb | |||||
| -Ataxic | -Urine microscopy for significant microscopic pyuria | ||||
| - GMFCS I-II (mild) | |||||
| -GMFCS IV-V (severe) | -A urine culture on sheep blood, MacConkey, and CLED agars | -Quantitative growth of bacteria > 105 colony forming units per ml urine, of the same organism (with > 1 UTI symptoms or signs)d | |||
| -GMFCS III (moderate) | |||||
| -Not intellectually | |||||
| -disabled | |||||
| -Intellectually-disabled | |||||
| Ryakitimbo et al. 2018 (14) | -Based on GMFCS | -Spastic quadriplegia | -A urethral catheter urine sample | -Urine dipstick for nitrite and leucocyte esterase | -Growth of a single uropathogen with at least 5 × 104 colony-forming units per microliter |
| -Spastic hemiplegia | |||||
| -Athetoid | |||||
| -Mixedb | |||||
| -Spastic diplegia | |||||
| -Dystonia | -Urine culture on MacConkey agar and 5% blood agar incubated at 37oC | ||||
| -GMFCS IV-V (severe) | |||||
| -GMFCS I-II (mild) | |||||
| -GMFCS III (moderate) |
Abbreviations: CLED, cysteine lactose electrolyte deficient; GMFCS, gross motor function classification system (grades severity of cerebral palsy); UTI, urinary tract infection; ZMD Quotient, Ziler’s Man-Drawing Quotient (grades the intellectual ability of children with cerebral palsy)
aListed in order of greater frequency as recorded in the study
bPyramidal and extrapyramidal cerebral palsy
cAsymptomatic bacteriuria or asymptomatic UTI
dSymptomatic UTI
3.3. Prevalence of UTI in Children with Cerebral Palsy
In Table 3, UTI prevalence rates reported in six studies (5, 12, 14-17) among a total of 360 children and adolescents with cerebral palsy were 2.2% (12), 48.1% (5), 32.1% (16), 56.7% (17), 38.5% (15), and 13.1% (14). One study which investigated the frequency of common infectious diseases in 60 children with disability, reported a UTI prevalence of 13.3% (13), although only 11 subjects had cerebral palsy. The estimated mean UTI prevalence for the six studies was 31.8%.
| Source | Prevalence of UTI | Subgroup Differences in UTI Prevalence | Bacterial Isolates (% Frequency) | Antibiotic-Sensitivity Patterns |
|---|---|---|---|---|
| Hellquist et al. 1985 (12) | 2.2% | -Not specified | -Not stated | -Not stated |
| Reid et al. 1993 (5) | 48.1% | -No previous UTI episode: 7.4% | -Not stated | -Not stated |
| -> 1 previous UTI episode: 40.7% | ||||
| Ozturk et al. 2006 (16) | 32.5% | -Enuretic group: 15.0 % | -Escherichia coli (30.8%); Proteus vulgaris (23.1%); Enterococcus faecalis (23.1%); Klebsiella oxytoca (7.7%); Staphylococcus saprophyticus (7.7%); Mixed growth of Proteus vulgaris and Serratia marcencens (7.7%) | -Not stated |
| -Non-enuretic group: 25.0% | ||||
| Silva et al. 2009 (17) | 56.7% | -Wheelchair group: 45.9% | -Mixed growth of Proteus and Enterococcus (50.0%) | -Not stated |
| -Walking group: 10.8% | -Streptococcus (50.0%) | |||
| Anigilaje et al. 2013 (15) | 38.5% | -Males: 23.1%; Females: 15.4% | -Escherichia coli (45.0%); Streptococcus faecalis (20.0%); Staphylococcus aureus (15.0%); Proteus spp. (10.0%); Klebsiella spp. (10.0%) | -Escherichia coli; ciprofloxacin, ofloxacin, sparfloxacin, ceftriaxone (100%), gentamycin (50% - 66.7%) but resistant to amoxiclav, cotrimoxazole, nitrofurantoin, tetracycline and nalidixic acid |
| -< 5 years: 0%; > 5 years: 38.5% | ||||
| -GMFCS I-II: 3.8%; GMFCS III-V: 34.6% | ||||
| -Enuretic group:19.2%; Non-enuretic group: 19.2% | ||||
| -Streptococcus faecalis, Staphylococcus aureus, Proteus spp. & Klebsiella spp.; quinolones and ceftriaxone (100%), streptomycin (33.3% - 50%), gentamycin (33.3%-75%) but resistant to amoxiclav, cotrimoxazole, nitrofurantoin, tetracycline and nalidixic acid | ||||
| Fahimzad et al. 2013 (13) | 13.3% a | -Not stated | -Not stated | -Not stated |
| Ryakitimbo et al. 2018 (14) | 13.1% | -< 7 years: 12.1%; >7 years: 1.0% | -Escherichia coli (53.8%); Proteus mirabilis (23.1%); Klebsiella pneumoniaeb (7.7%); Staphylococcus aureus & Enterococcus faecalis (15.4%) | -Escherichia coli; ciprofloxacin & ceftriaxone (100%), gentamicin, amoxiclav & nitrofurantoin (85%), cefotaxime (71%), ampicillin & cotrimoxazole (28.6%) |
| -Males: 8.1%; females: 5.0% - | ||||
| -GMFCS I-II: 5.0%; GMFCS III: 1.0%; GMFCS IV-V: 7.1% | -Proteus mirabilis; ciprofloxacin, gentamicin & ceftriaxone (100%) | |||
| -E faecalis & Staph aureus; all tested antibiotics (100%) except ampicillin for Staph aureus |
Abbreviations: GMFCS, gross motor function classification system; UTI, urinary tract infection
aPrevalence among disabled children (majority had cerebral palsy)
bSensitive to all antibiotics (100%) except to ampicillin, cotrimoxazole & nitrofurantoin
Some studies highlighted sub-group differences in UTI prevalence (5, 14-17). In one study, children with cerebral palsy presenting with enuresis had a UTI prevalence of 15.0%, while those without enuresis had a rate of 25.0% (16). Three studies noted these major differences: UTI prevalence of 45.9% in the patients on wheel chair versus 10.8 % in those that were ambulant (17); UTI prevalence of 19.2% in those with enuresis versus 19.2% in those without enuresis, and prevalence of 3.8% in those with GMFCS I-II versus 34.6% in those with GMFCS III-V (15); and finally prevalence of 5.0% in those with GMFCS I-II versus 8.1% in GMFCS III-V group (14).
In Table 3, four studies reported the names and frequencies of the bacterial isolates cultured from the patients’ urine specimen (14-17). Three of these studies reported Escherichia coli as the most frequent uropathogen (14-16), while Proteus and Enterococcus in mixed growth were the predominant uropathogens documented in the remaining study (17). The antibiotic-sensitivity patterns of the most prevalent bacterial isolate (Escherichia coli) were reported by two studies. The uropathogen was noted to be 100% sensitive to quinolones and ceftriaxone in the two studies (14, 15). However, there were some differences in the antibiotic-sensitivity pattern of Escherichia coli in these studies. The organism was 50% - 66.7% sensitive to gentamycin but was resistant to amoxiclav, cotrimoxazole, nitrofurantoin, tetracycline and nalidixic acid in one of the studies (15). In the other study, Escherichia coli was 85% sensitive to gentamycin, amoxiclav, and nitrofurantoin, 71% sensitive to cefotaxime, and 28.6% sensitive to ampicillin and cotrimoxazole (14).
3.4. Risk of Bias Within Studies
In one study (12), the convenience sampling technique possibly introduced sampling bias. In the two retrospective cross-sectional studies (5, 17), confirmation bias was a likely study limitation given that subjects already had lower urinary tract symptoms before referral. For the prospective cross-sectional studies (13, 14), convenience sampling was also adopted with the likelihood of sampling bias in one study (14) while the other study (13) possibly had a selection bias given the enrolment of children with other forms of disability. Finally, the two prospective case-control studies (15, 16) reported subject drop-outs (attrition bias) and used non-random sampling with the likelihood of sampling bias.
3.5. Synthesis of Results
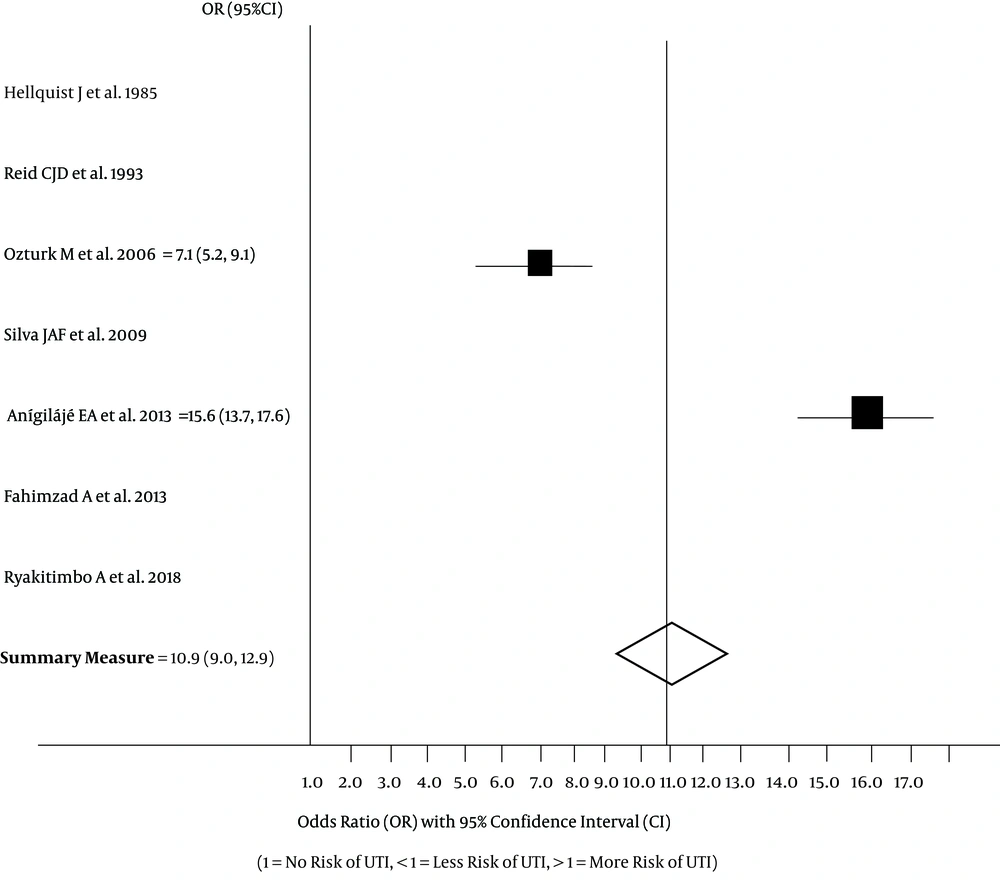
Only the two case-control studies (15, 16) had high precision. None of the six studies (5, 12, 14-17) reported OR values to assess UTI risk in affected children. The log OR at 95% CI was, however, estimated for the case-control studies, giving a value of 10.9 (95% CI: 9.0, 12.9). In the forest plot displayed in Figure 2, one study had a UTI OR of 7.1 (95% CI: 5.2, 9.1) (16) and the other, an OR of 15.6 (95% CI:13.7, 17.6) (15). These two studies therefore suggest that children and adolescents with cerebral palsy have significantly high risk for UTI. The findings indicate that the probability of UTI developing in these patients is very high when compared to their counterparts without this neurologic disorder.
3.6. Risk of Bias Across Studies
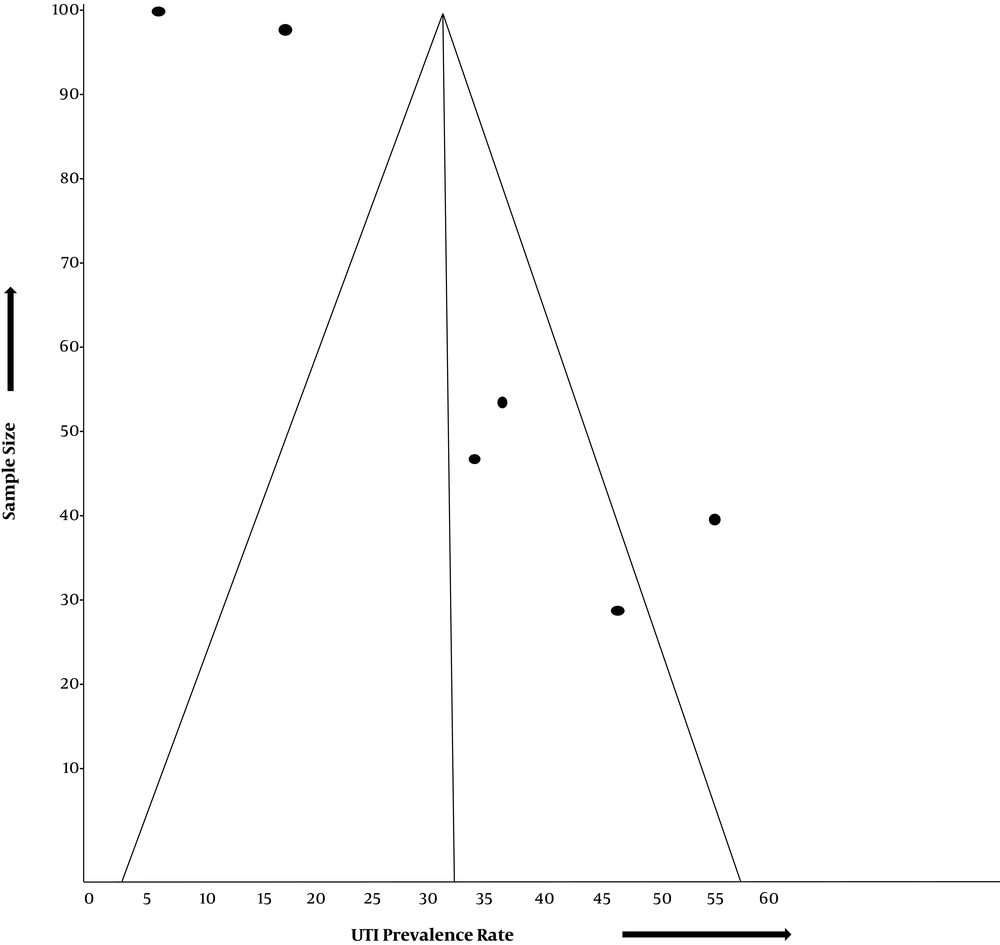
The funnel plot assessing for publication bias across six studies shows a deviation from the normal pattern (Figure 3). The case-control studies (15, 16) appear within the funnel, closer to the vertical midline of the funnel, which represents the mean UTI prevalence rate estimated for the six studies. They also appear nearer to the funnel apex than the funnel base, signifying their higher precision. Three studies (12, 14, 17) appear completely outside the funnel, while one study (5) appears within the funnel but farther away from the vertical midline.
4. Conclusions
Children and adolescents with cerebral palsy may be susceptible to UTI. Some published studies have confirmed high prevalence rates of the infection in these patients. Few studies however reported low prevalence rates. This disparity in UTI prevalence rates may suggest the absence of a strong evidence to support routine screening and treatment for the infection in children and adolescents with this disorder. A systematic review that estimates the pooled UTI prevalence rate from these studies can establish if the UTI burden in these patients is significant enough to justify this management approach.
In this review, we calculated the mean prevalence rate from six studies and odd ratio from two studies, and found that children and adolescents with cerebral palsy have a risk for UTI and a high prevalence rate compared to their healthy counterparts. The estimated mean prevalence of 31.8% agrees with the rates of 38.5% and 32.1% reported in the case-control studies (15, 16), that were considered as high quality studies based on qualitative analysis. Furthermore, the mean UTI prevalence rate in our review is comparatively higher than the pooled UTI prevalence of 17% in malnourished children (10), UTI point prevalence of 5.8% - 21.6% in children with sickle cell anemia (24-26) and 10.3% in HIV-infected children (27). Given the suggestion to incorporate the screening and treatment for UTI in malnourished children based on the relatively lower prevalence rate of 17% (10), the argument that favors a similar management protocol in children with cerebral palsy may also be justified. The pathophysiologic mechanisms which support the predisposition of these children to UTI are well documented. For instance, cerebral palsy is associated with lower urinary tract dysfunction, especially symptomatic neurogenic bladder (5, 17, 28-30). The increased bladder capacity, high voiding pressures, and high residual volumes in a neurogenic-bladder model predispose the patients to UTI (31). Risk factors for UTI in the neurogenic bladder also include some intrinsic bladder mechanisms and immunologic variations (32, 33). Urothelial cell apoptosis, exfoliation and impairment of barrier-function urothelium, and differences in secretory IgA between the normal bladder and neurogenic bladder all predispose to UTI (33). Secondly, urinary continence develops with the maturation of cortical pathways in children, which regulate the micturition center from where the micturition reflex is inhibited. Cerebral lesions that blunt this inhibition will lead to detrusor overactivity and voiding dysfunction (34). Thus, UTI risk in children with cerebral palsy may arise from vesicoureteral reflux (VUR) and incomplete bladder emptying triggered by detrusor overactivity and detrusor-sphincter dyssynergia (2, 3, 5, 35). Additionally, urinary retention (due to immobility and impaired cognitive ability to communicate bladder fullness and micturition urge) contribute to the infection risk (13).
Our systematic review also found higher UTI prevalence rates in subgroups of cerebral palsy patients with gross motor limitation, such as those on wheelchair [45.9%] compared to those that were ambulant (10.8%) (17), and those with GFMCS III-V [34.6%] compared to those with GFMCS I-II (3.8%) (15); these findings further underscore the role of neurogenic bladder and urinary retention from immobility or impaired cognitive ability in predisposing these patients to UTI. More importantly, they indicate a greater contribution to UTI burden by this category of patients with cerebral palsy. Thus, these patients may require a much earlier intervention for infection screen and treatment. Again, we noted that Escherichia coli was the commonest etiologic agent in most studies (14-16), while one study reported mixed growth of Proteus and Enterococcus as the predominant organisms (17). Whereas UTI in a normal bladder is caused by Escherichia coli and Klebsiella spp, the same infection in a neurogenic bladder is usually caused by Enterococcus, Pseudomonas aeruginosa and a mixed growth of uropathogens (36). These findings are in tandem with the observations from the studies we reviewed. These studies most likely investigated cerebral palsy patients with normal bladder (14-16) and those with neurogenic bladder (17). Although UTI etiologic patterns should ideally guide the choice of empirical antibiotic treatment, it may be preferable to conduct a pre-treatment urine culture because of the following reasons. Firstly, we noted unpredictable antibiotic-sensitivity patterns for the predominant uropathogens reported in the reviewed studies, making pre-treatment urine cultures a fundamental prerequisite for effective eradication of UTI in children and adolescents with cerebral palsy. Secondly, the increasing prevalence of extended-spectrum β-lactamase-producing bacteria has highlighted the problem of multi-antibiotic resistance. This trend calls for rational antibiotic therapy and discourages routine empirical antibiotics in these patients.
4.1. Limitations
The few numbers of reviewed studies are a limitation for applying a robust meta-analysis. Although our funnel plot shows a deviation (probably suggesting a publication bias in the included studies), it could also have been due to other factors such as an inappropriate effect measure, as well as a systematic difference between higher-precision studies (15, 16), and lower-precision studies (5, 12, 14, 17). Unfortunately, only the two higher-precision studies fulfilled the methodological quality required to estimate a summary measure. Thus, the calculated OR that indicates a significant UTI risk in pediatric patients with cerebral palsy may have to be generalized with caution.
4.2. Recommendation
Given the paucity of higher-precision studies conducted so far on the risk and prevalence of UTI in children and adolescents with cerebral palsy, we recommend more prospective case-control studies. The findings of such studies will raise the level of evidence and form the basis for a practice guideline for managing UTI in these patients. A repeat systematic review may be required in future when more of such studies are conducted. Nevertheless, the current systematic review has shown that the high burden of UTI reported in children and adolescents with cerebral palsy may be a justification for screening and treating the infection routinely in these patients.
The review was registered on 4th April 2020 with PROSPERO (Acknowledgement receipt number-178323)