1. Background
Congenital malformations are structural or functional anomalies at birth that lead to physical, mental, and developmental disabilities (1, 2). Genetic and environmental factors, as well as a combination of them, may cause congenital malformations. Appropriate diagnostic and therapeutic tools have gradually improved over the past decades and helped us to identify better and reduce the long-term effects and mortality. Early identification of congenital malformations is the first step to providing useful genetic counseling for parents. Nowadays, due to the importance of life expectancy in newborns, congenital malformations are the most crucial issue in health care (3). Annually, an average of 3 - 6% of newborns, about 8 million babies worldwide, are born with a severe congenital malformation, and estimates show that more than 90% of these babies are born in low- and average-income countries (4). Congenital malformations can occur as a defect or a combination of defects (5). Research showed that about 65 - 75% of congenital malformations are multifactorial. According to the results of several studies, factors such as defects in one or more genes (6), hereditary factors (7), diabetes mellitus (8-10), mother’s age (11), mother’s living environment during pregnancy (12), and consanguineous marriage (13-15) are the influencing factors for congenital malformations.
In a retrospective study by Verma et al. (1991) conducted on 10,000 babies born between January 1983 and March 1989, the prevalence of congenital malformations was reported as 6.6%. In this study, most of the anomalies were due to central nervous system (CNS). Anomalies were similar in both genders, although genital anomalies were more common in boys (16). Some chronic diseases like diabetes mellitus and high blood pressure in mothers are known as risk factors for many congenital malformations (17, 18).
2. Objectives
This study aimed to examine the factors affecting the birth of infants with congenital malformations using logistic regression.
3. Methods
3.1. Patient Population
We conducted this study based on the data of neonatal malformations registered in Iranian Maternal and Neonatal Network (IMAN). In this retrospective descriptive-analytical study, we analyzed the information of all live births and their mothers in 2015 in maternity hospitals of Iran in terms of variables such as gender, birth weight of the baby, consanguineous marriage, location of residence, chronic and underlying maternal diseases, and type of delivery. The study was approved by the Ethical Committee of Tabriz University of Medical Sciences (IR.TBZMED.REC.1399.688).
3.2. Data Collection
In this study, the information was analyzed based on all severe congenital malformations, including hands, feet, and nervous system malformations, gastrointestinal disorders, and genital malformations. Data gathering was performed using a checklist.
3.3. Statistical Analyses
Data analysis was done using chi-square test to investigate the associations between different factors and factors affecting congenital malformations. Logistic regression has been used by SPSS-26 software. P-value < 0.05 was considered as statistically significant.
4. Results
4.1. Descriptive Statistics
Out of a total of 1,491,883 newborns and their mothers, 111,211 (7.5%) babies were born with congenital malformations. Most of these infants had one or two anomalies (83.77% had one malformation, and 10.80% had two malformations). Also, 768,782 (51.6%) infants were male and 722,416 (48.4%) were female. Table 1 shows the information about newborns and their mothers.
| Variable | No. (%) |
|---|---|
| Chronic blood pressure | |
| Yes | 16076 (1.1) |
| No | 1475807 (98.9) |
| Eclampsia | |
| Yes | 22330 (1.5) |
| No | 1469553 (98.5) |
| Diabetes mellitus | |
| Yes | 39152 (2.6) |
| No | 1452731 (97.4) |
| Consanguineous marriage | |
| Yes | 1175211 (78.8) |
| No | 316672 (21.2) |
| Place of residence | |
| Rural | 371006 (24.9) |
| Urban | 1120877 (75.1) |
| History of abortion | |
| Yes | 255240 (17.1) |
| No | 1236643 (82.9) |
a Diabetes variables include all mothers with type 1 diabetes and type 2 diabetes.
The following tables show the associations between the variables of chronic blood pressure, eclampsia, diabetes, gender, history of abortion, and place of residence of parents with a malformation based on the chi-square test. As can be seen in Tables 2 to 8, all the variables had a significant relationship with congenital malformations, except chronic blood pressure (P-value < 0.05).
| Chronic Blood Pressure, Count (%) | Test Results | ||||
|---|---|---|---|---|---|
| No | Yes | χ2 | df | P-Value | |
| Congenital malformation | 0.970 | 1 | 0.325 | ||
| No | 1365827 (98.9) | 14845 (1.1) | |||
| Yes | 109980 (98.9) | 1231 (1.1) | |||
| Eclampsia, Count (%) | Test Results | ||||
|---|---|---|---|---|---|
| No | Yes | χ2 | df | P-Value | |
| Congenital malformation | 37.151 | 1 | < 0.001 | ||
| No | 1360244 (98.5) | 20428 (1.5) | |||
| Yes | 109309 (98.3) | 1902 (1.7) | |||
| Diabetes Mellitus, Count (%) | Test Results | ||||
|---|---|---|---|---|---|
| No | Yes | χ2 | df | P-Value | |
| Congenital malformation | 48.309 | 1 | < 0.001 | ||
| No | 1344795 (97.4) | 35877 (2.6) | |||
| Yes | 107936 (97.1) | 3275 (2.9) | |||
| Abortion history, Count (%) | Test Results | ||||
|---|---|---|---|---|---|
| No | Yes | χ2 | df | P-Value | |
| Congenital malformation | 69.666 | 1 | < 0.001 | ||
| No | 1145467 (83.0) | 235205 (17.0) | |||
| Yes | 91176 (82.0) | 20035 (18.0) | |||
| Consanguineous Marriage, Count (%) | Test Results | ||||
|---|---|---|---|---|---|
| No | Yes | χ2 | df | P-Value | |
| Congenital malformation | 85.780 | 1 | < 0.001 | ||
| No | 1088821 (78.9) | 291851 (21.1) | |||
| Yes | 86390 (77.7) | 24821 (22.3) | |||
| Place of Residence, Count (%) | Test Results | ||||
|---|---|---|---|---|---|
| Urban | Rural | χ2 | df | P-Value | |
| Congenital malformation | 202.698 | 1 | < 0.001 | ||
| No | 345324 (25.0) | 1035348 (75.0) | |||
| Yes | 25682 (23.1) | 85529 (76.9) | |||
| Gender, Count (%) | Test Results | ||||
|---|---|---|---|---|---|
| Boy | Girl | χ2 | df | P-Value | |
| Congenital malformation | 13.304 | 1 | < 0.001 | ||
| No | 710999(51.5) | 669251 (48.5) | |||
| Yes | 57783 (52.1) | 53165 (47.9) | |||
4.2. Logistic Regression
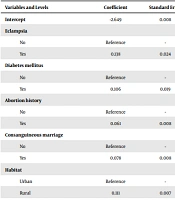
After examining the relationship between independent variables and dependent variables (congenital malformations), eclampsia, diabetes mellitus, consanguineous marriages, place of residence, gender, and history of abortion entered the logistic regression model (Table 9).
| Variables and Levels | Coefficient | Standard Error | P-Value | Odds Ratio | 95% CI for Odds Ratio |
|---|---|---|---|---|---|
| Intercept | -2.649 | 0.008 | 0.000 | 0.07 | - |
| Eclampsia | |||||
| No | Reference | - | - | - | - |
| Yes | 0.138 | 0.024 | 0.000 | 1.15 | (1.095, 1.204) |
| Diabetes mellitus | |||||
| No | Reference | - | - | - | - |
| Yes | 0.106 | 0.019 | 0.000 | 1.11 | (1.072, 1.153) |
| Abortion history | |||||
| No | Reference | - | - | - | - |
| Yes | 0.061 | 0.008 | 0.000 | 1.06 | (1.046, 1.080) |
| Consanguineous marriage | |||||
| No | Reference | - | - | - | - |
| Yes | 0.078 | 0.008 | 0.000 | 1.08 | (1.066, 1.098) |
| Habitat | |||||
| Urban | Reference | - | - | - | - |
| Rural | 0.111 | 0.007 | 0.000 | 1.12 | (1.101, 1.133) |
| Gender | |||||
| Female | Reference | - | - | - | - |
| Male | 0.022 | 0.006 | 0.000 | 1.02 | (1.010, 1.035) |
We analyzed the cause of birth anomalies by logistic regression analysis as the dependent variable, and the variables of eclampsia, diabetes mellitus, consanguineous marriage, place of residence, gender, and history of abortion were predictive (independent) variables. A total number of 1,491,883 neonates entered the analysis, and the full model was significant (χ2 = 456.250, df = 7, P-value < 0.001). The results showed that the variables of eclampsia, diabetes, consanguineous marriage, place of residence, infant’s gender, and history of abortion significantly predicted the infants’ congenital malformations. The chance of having a baby with congenital malformation was 15% higher in mothers with eclampsia than in healthy mothers, and 11% higher in mothers with diabetes mellitus than in healthy mothers. Also, the chance of having a baby with congenital malformations in rural areas was 12% higher than in urban areas. A history of abortion, consanguineous marriage, and the infant’s gender were factors influencing the onset of congenital malformations, although the odds ratios (OR) for these variables were close to 1.
5. Discussion
There are several influencing factors for congenital malformations, including chronic maternal illnesses such as diabetes mellitus, eclampsia, a history of abortion, and consanguineous marriages. These factors increase the chance of congenital malformations in babies. Moreover, in rural areas, the rate of congenital malformations was higher than urban areas. This may be due to the lack of facilities, regular tests, and ultrasounds, which indicates more serious attention for planning services of premarital counseling, testing, controls, and health services during pregnancy in rural areas. Also, male infants were more likely to have congenital malformations than female ones. In a study by Verma et al., maternal factors such as previous abortions, drug abuse, fever in the first trimester of pregnancy, diabetes mellitus, eclampsia, and anti-drip bleeding had a significant association with congenital malformations in infants. Our study showed a significant association between factors such as diabetes mellitus, eclampsia, and previous maternal abortions with congenital malformations in infants, which is consistent with the results of the study by Verma et al. In the study conducted by Verma et al., the malformations were similar in both genders, but in our study male infants had more malformations (16). In a multicenter case-control study in 2008, Correa et al. used data from approximately 18,000 deliveries from October 1997 to December 2003. In their study, there was a strong association between diabetes mellitus and congenital malformation, which is in line with our study results (17). In a 2012 cross-sectional study, Lin et al. concluded that the prevalence of congenital malformation in urban areas was higher than in rural areas, which is inconsistent with the results of our study (19). Kar et al. (2018), in a non-interventional hospital-based clinical trial study gathered data from September 2015 to August 2016 to analyze the prevalence of congenital malformation and the factors affecting it. They concluded that one of the factors influencing the incidence of congenital malformations is living in rural places, which is consistent with the results of our study (20). In a 2016 study in northern Iran, Kaviany et al. concluded that congenital malformations were significantly related to consanguineous marriages, which was similar to our study (21).
In a 2016 review study, Ng (22) found that consanguineous marriages may increase the chance of getting congenital malformation. Also, in a cross-sectional study conducted on 138 married couples and their children in 2016 by Al-Joborae et al., the prevalence of congenital malformations was significantly higher for parents with relatives (especially close relatives such as cousins) than the stranger parents (23). The results of these studies are similar to those obtained in the present study.
Lary and Paulozzi studied the prevalence of congenital malformations and concluded that male infants were more at risk for congenital malformations than females, which is in line with our study (24). In a 2014 descriptive-analytical study, Amini Nasab et al. examined the data of 118 infants from 2007 to 2011. Their results showed that congenital malformation was more common in male infants (55.9%) than in females (44.1%) (25). These results are also similar to our results.
Although chronic blood pressure is one of the most important and influential factors in the birth of babies with congenital malformations (18, 26, 27), we did not witness the effect of this factor in our study. Bellizzi et al. (2016) analyzed data from the World Health Organization (WHO) multi-country survey in which they reported 310,401 babies from 359 centers in 29 countries. They used logistic regression model with a random effect for detecting associations between six widespread congenital malformations and four high blood pressure disorders in mothers in the form of chronic blood pressure, preeclampsia, eclampsia, and chronic hypertention. This study showed that high blood pressure in mothers significantly increased the risk of congenital malformations of the kidneys, limbs, and lips/cleft/palate (18).
5.1. Conclusions
Our findings suggest that such measures as premarital counseling, regular pre-pregnancy and post-pregnancy tests, and controls, especially in rural and deprived areas, are essential to reduce the incidence of congenital malformations in Iran.