1. Background
Heart failure (HF) is an impairment in the ability of the ventricle to fill with or eject blood due to structural or functional issues. This disorder leads to problems in hemodynamics, oxygen consumption, and exercise capacity and eventually becomes pathologic for almost every organ in the body (1).
The vasoactive-ventilation-renal (VVR) score is a novel disease severity index which includes components reflecting cardiovascular, pulmonary, and renal function. Recently, the usefulness of the VVR score in pediatric patients undergoing cardiac surgery has been reported (2, 3). In some studies (4, 5), the VVR score was shown to be predictive in particular of a prolonged length of stay (PLOS) in the intensive care unit (ICU).
The lactate level has been shown to be associated with the LOS in the ICU or hospital in adult patients who have undergone cardiac surgeries (6, 7). In addition, brain natriuretic peptide (BNP) was previously found to be associated with PLOS in adult HF patients (8). Vasoactive-inotropic score (VIS) was shown to be associated with PLOS in ICU in infants receiving cardiac surgery (9).
2. Objectives
In the current study, we aimed to determine whether the VVR score can predict pediatric ICU (PICU) PLOS in children with HF. We also aimed to compare the usefulness of the VVR score with the lactate or BNP levels in relation to these predictive properties.
3. Methods
We conducted a retrospective cohort study of children with HF patients admitted to the PICU of Asan Medical Center Children's Hospital, Seoul, Republic of Korea, between January 2010 and December 2016. All patients included in the study were admitted to the medical section of PICU, and those with postoperative problems of any kind were excluded. Patients who had undergone heart transplantation or died were excluded, and only the children who had survived to discharge or transfer to the general ward were analyzed. Following our own clinical protocols, laboratory tests including lactate and BNP were immediately done, and echocardiograms were conducted by the attending intensivist or a cardiologist for all PICU cases on admission. The diagnosis of HF was made when all the following criteria were met: (1) the presence of clinical symptoms of HF (dyspnea, feeding difficulty, diaphoresis, etc.); (2) echocardiographic evidence of systolic or diastolic dysfunction; and (3) the BNP level at admission greater than 100 pg/mL (10). We excluded any children who did not meet the above diagnostic criteria based on a medical record review or for whom complete echocardiographic or laboratory test data were not available. This study was approved by the Institutional Review Board of Asan Medical Center (S2019-2723-0001).
Patient demographics and data on the length of PICU stay, HF etiology, classification of HF, and need for mechanical ventilation/mechanical circulatory support by extracorporeal membrane oxygenation (ECMO)/renal replacement therapy were collected. PLOS was defined as a value greater than the median LOS for all of our HF patients. HF was classified according to the New York Heart Association (NYHA) guidelines for older children and Ross HF classification in younger children. The VIS was calculated as the dopamine dose (mcg/kg/min) + dobutamine dose (mcg/kg/min) + 100 × epinephrine dose (mcg/kg/min) + 10 × milrinone dose (mcg/kg/min) + 10,000 × vasopressin dose (units/kg/min) + 100 × norepinephrine dose (mcg/kg/min). The VVR was calculated as ventilation index + VIS + Δ creatinine (change in creatinine from baseline × 10). Ventilator index was calculated as the PaCO2 (mmHg) × peak airway pressure (cmH2O) × respiratory rate (breaths/min)/1,000. Concerning the ventilation index, the maximum measurement during the first 6 hours after admission to the PICU was used; if a patient did not receive mechanical ventilation, the ventilation index was defined as ‘0’. The inotrope (s) dose and creatinine measurements were obtained from the measurements first recorded at the time of PICU admission.
Statistical analysis was performed using SPSS software for Windows® version 21 (SPSS, Chicago, IL). Continuous variables were analyzed using the Wilcoxon rank sum test, and presented as a median [interquartile range]. Cross tabulation analysis was performed to analyze the correlation between mechanical life support application and PLOS, and an odds ratio (OR) was presented. Receiver operating characteristic (ROC) curves were created to analyze the usefulness of different clinical parameters in predicting the possible need for mechanical life support application or PLOS. In all analyses, a P-value of less than 0.05 was considered statistically significant.
4. Results
During the study period, 113 children with HF were admitted to our PICU. Twelve of these patients died, and five received heart transplantation and were thus excluded from further analysis. A final sample population of 96 children with HF was finally included in the present study. The median age of the included patients was 0.5 years, and 44 (45.8%) were male. The median PICU LOS was 12 days (IQR 6, 22). Fifty-six (58.3%) of these children required mechanical ventilation, 15 (15.6%) required ECMO, and 11 (11.5%) required renal replacement therapy (Table 1). The baseline characteristics (age, sex, HF etiology, classification of HF, and application of mechanical life support) were not significantly different between the PLOS group versus no-PLOS group (table not shown).
| Characteristics | n = 96 |
|---|---|
| Age (y) | 0.5 [0.2, 6.9] |
| Sex, male | 44 (45.8) |
| Length of pediatric intensive care unit stay, days | 12 [6, 22] |
| HF etiology | |
| Congenital heart disease/repaired | 52 (54.2) / 38 (39.6) |
| Cardiomyopathy | 24 (25.0) |
| Myocarditis | 15 (15.6) |
| Arrhythmia | 3 (3.1) |
| Rejection of transplanted heart | 2 (2.1) |
| Classification of heart failure | |
| Class III | 10 (10.4) |
| Class IV | 86 (89.6) |
| Mechanical ventilation | 56 (58.3) |
| ECMO | 15 (15.6) |
| Renal replacement therapy | 11 (11.5) |
Clinical and Demographic Characteristics of the Study Patients a
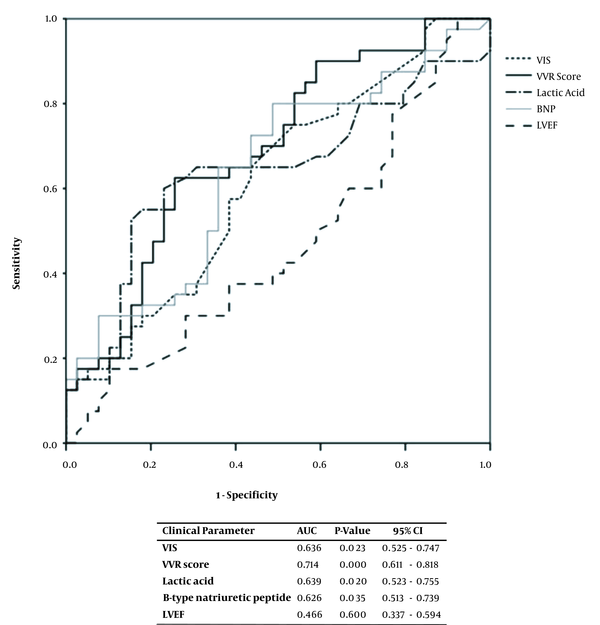
The children were divided into PLOS and no-PLOS groups, and their clinical parameters were compared. The VIS, VVR score, lactic acid levels, and BNP concentrations were all greater in the PLOS group (Table 2). The association between the clinical parameters and the PLOS was analyzed using ROC curves, and the VIS, VVR score, lactic acid levels, and BNP amounts were found to have a statistically significant relationship, each yielding an area under the curve (AUC) of 0.636, 0.714, 0.639, and 0.626, respectively (Figure 1).
| Variables | No-PLOS (n = 48) | PLOS (n = 48) | P-Value |
|---|---|---|---|
| VIS | 8.5 [5.0, 18.3] | 14.1 [7.4, 24.3] | 0.018 |
| VVR score | 11.1 [5.5, 20.7] | 27.8 [11.6, 36.2] | < 0.001 |
| Lactic acid (mmol/L) | 1.8 [1.1, 2.5] | 2.8 [1.2, 5.3] | 0.020 |
| B-type natriuretic peptide (pg/mL) | 1478.5 [529.8, 4532.8] | 2340.0 [1234.8, 6836.8] | 0.036 |
| Left ventricular ejection fraction (%) | 44.5 [27.8, 67.0] | 37.0 [21.5, 63.8] | 0.593 |
Clinical Parameters at Admission in the PLOS and No-PLOS Groups a
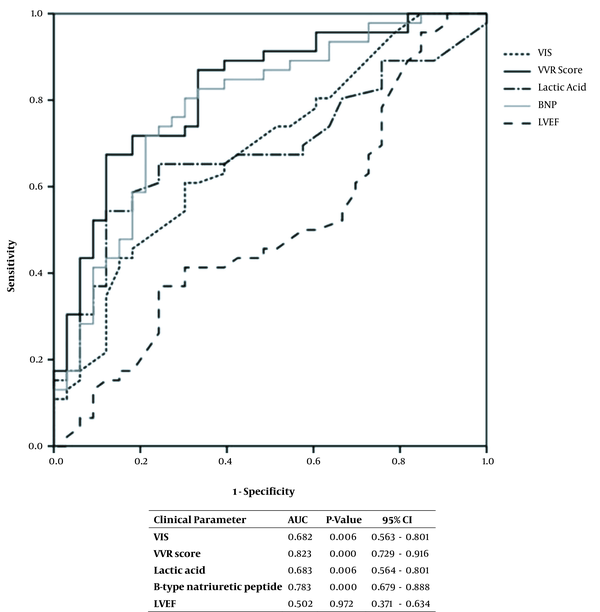
The requirements for the use of a mechanical ventilator and ECMO were found to correlate with a PLOS, with OR of 10.82 and 3.61, respectively (Table 3). The ROC curve analysis of the association between clinical parameters of interest and the need for a mechanical ventilator or ECMO showed statistically significant results for the VIS and VVR values and lactic acid and BNP levels each yielding an AUC of 0.682, 0.823, 0.683, and 0.783, respectively (Figure 2). When the cutoff for the VVR score was set as ≥ 10, the sensitivity, specificity, positive predictive, and negative predictive values for the PLOS were 82.6, 50.0, 60.3, and 75.8%, respectively (Table 4). Multivariable logistic regression analysis revealed that the VVR score was the only significant predictor of PLOS (Table 5).
| Variables | No-PLOS (n = 48) | PLOS (n = 48) | Odds Ratio | P-Value |
|---|---|---|---|---|
| Mechanical ventilator | 16 (33.3) | 40 (83.3) | 10.82 | < 0.001 |
| ECMO | 4 (8.3) | 11 (22.9) | 3.61 | 0.048 |
| RRT | 3 (6.3) | 8 (16.7) | 3.30 | 0.111 |
Correlation Between the Requirement for a Mechanical Ventilator/RRT/ECMO Intervention and PLOS a
| Variables | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| Prolonged length of stay | 82.6 | 50.0 | 60.3 | 75.8 |
| Mechanical ventilator/ECMO | 85.7 | 62.5 | 76.2 | 75.8 |
Predictability of the VVR Score for PLOS and Mechanical Ventilator/ECMO Interventions when the Cutoff was Set at ≥ 10
| Variables | Odds Ratio | 95% Confidence Interval | P-Value |
|---|---|---|---|
| VIS | 0.920 | 0.839 - 1.009 | 0.075 |
| VVR score | 1.091 | 1.016 - 1.172 | 0.016 |
| Lactic acid | 1.112 | 0.920 - 1.346 | 0.273 |
| B-type natriuretic peptide | 1.000 | 1.000 - 1.000 | 0.543 |
| Left ventricular ejection fraction | 0.996 | 0.972 - 1.020 | 0.730 |
Multivariate Logistic Regression Analysis for Predictors of PLOS
5. Discussion
Based on our findings, the VVR score at admission is significantly higher in children with HF who will eventually require a PLOS in the PICU. In addition, the application of a mechanical ventilator or ECMO in these cases showed an association with a PLOS, and the VVR score was the best predictor of the requirement for these interventions.
A longer hospitalization for HF treatment was previously reported to be associated with poorer patient outcomes (11, 12). This prompted efforts to elucidate predictive or associated factors with a longer LOS in HF patients. Omar HR et al. identified higher BNP levels in their longer-than-average LOS group (13). In our study, we used the median LOS as the cutoff for defining PLOS. The BNP level was a significant factor in univariable analysis for predicting PLOS, but not in subsequent multivariable analysis in which VVR score was the only significant predictor. Consistently, further ROC curve analysis revealed that the AUC of the VVR score was the largest among the tested parameters.
Organ impairment is frequently observed in HF patients. HF also leads to congestion and hypoperfusion, which can eventually lead to the failure of other organs such as the lung and kidney (14). Mechanical life support systems such an ECMO, ventilator, and renal replacement therapy (RRT) are required in these cases (15). In previous studies, the application of mechanical ventilators was shown to be associated with an ICU PLOS (16, 17), and ECMO interventions were shown to further increase this duration (18, 19). Similarly in our present study in children with HF, the need for mechanical ventilation or ECMO was associated with a PLOS in the PICU. Because the VVR score is a calculation formula consisting complementary pulmonary and renal components in addition to VIS, it is theoretically and clinically plausible that the greater the VVR score, the need for not only ECMO but also mechanical life support system like ventilator and RRT increases. Based on our findings, the VVR score was found to be the best predictor of the requirement for this support, which likely underlies its superior predictive properties in relation to PLOS.
Our present study had some limitations of note, principally arising from the retrospective nature of the analyses. In addition, the administration of vasoactive medication or the degree of mechanical ventilation is likely to have varied according to the judgment and practices of the attending physician. However, the intensivist team members at our hospital who treated these children were unchanged during the study period, which would have minimized the bias in terms of clinical decision making processes. Another shortcoming of our current report was the small sample size. Future prospective studies of larger cohorts are warranted to validate our preliminary findings, and further elucidate the factors that may affect the VVR score under a consistent practice protocol.
Another hypothesis is that the VVR score at PICU admission would be associated with a higher risk for major adverse outcomes like death and heart transplantations. During the study period, 12 patients died, and five patients received heart transplantations, so the small numbers presenting major adverse outcomes restricted us from conducting supplementary analysis on this issue. Future studies, including a larger sample size are needed to further analyze the detailed variables of major adverse outcomes.
In conclusion, the VVR score for pediatric HF patients at admission to the PICU is a useful predictor of the need for mechanical ventilator or ECMO support. Since mechanical support interventions correlate with PLOS in such cases, the VVR score at admission can also be a useful predictor of an extended stay in the PICU for pediatric HF patients.