1. Context
Deciduous teeth, commonly known as baby teeth or milk teeth, play a peculiar role in beauty, nutrition, speech, normal function, projected growth and space retention for permanent teeth (1). According to the American Academy of Pediatric Dentistry, early childhood caries (ECC) is considered as one or more decayed, missing, or filled tooth surfaces in any primary tooth in children less than 6 years of age (2). Severe early childhood caries (S-ECC) is defined as smooth-surface caries in a child younger than 36 months of age (3). Not only does this disorder present a dental problem but it also stunts Children’s growth due to insufficient food intake to meet developmental and metabolic needs. Therefore, children are arguably among the most vulnerable groups in the early years of life (2). The prevalence of ECC has been reported as 1% - 12% and 70% in developed and less developed countries, respectively (4). The consequences of ECC affect the quality of life of children and their families and can have adverse socio-economic consequences (5). In addition to microbial etiology, various factors, such as a diet containing fermentable carbohydrates, genetical, immunological, behavioral, and environmental factors (e.g., social, economic, and cultural) and baby bottle syndrome, which is one of the most common types of rampant caries in children younger than 6 years of age, can also influence the prevalence of caries (6, 7).
ECC salivary biomarkers are classified into three categories: biological compounds, physical properties, and chemical compounds of saliva (8). Saliva is a colorless, odorless hypotensive solution with a relative density of 1.004 - 1.009 and a pH of 6.6 - 7.1, which is secreted from the acinar cells of the salivary glands, gingival fluid, and oral mucosa. Approximately 90% of saliva is secreted by the salivary glands which are surrounded by numerous capillaries. Therefore, the biomarkers in the bloodstream are easily infiltrated into acinar cells and secreted into the saliva. There are more than 700 types of microorganisms in saliva that are associated with systemic and oral diseases (9). According to a study conducted by Samaranayake, there are more than 1 × 106 mL-1Streptococcus mutans and/or 1 × 105 mL-1 Lactobacillus in the saliva of people with high caries activity. On the other hand, less than 1 × 105 mL-1Streptococcus mutans and/or 1 × 104 mL-1 Lactobacillus were detected in the saliva of people with low caries activity (10). One of the salivary biomarkers is nitric oxide (NO) which is synthesized either chemically (by dietary nitrate metabolism) (11) or enzymatic (by L-arginine decomposition with NO synthase enzyme secreted from salivary glands and other tissues) (12).
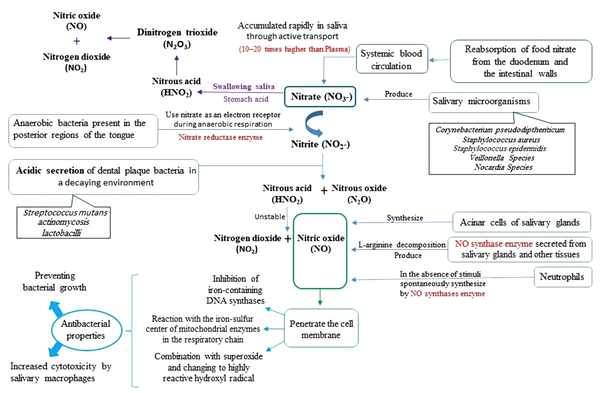
Typically, saliva contains 1,500 micromoles nitrate (NO3-) and 100 micromoles nitrite (NO2-) (13, 14). Consequently, salivary nitrate levels are 10 - 20 times higher than those levels found in plasma. In the human body, nitrate is a neutral substance and no enzyme exists to convert it. In the oral cavity, saliva nitrate is reduced to nitrite in contact with anaerobic bacteria present in the posterior regions of the tongue by the action of nitrate reductase enzyme during anaerobic respiration. The acidic secretion of dental plaque bacteria (e.g., Streptococcus mutans, lactobacilli, and actinomycosis) in a decaying environment leads to the acidification of nitrite and the formation of nitrous acid. Nitrous acid is an unstable acid that spontaneously decomposes and produces a combination of nitrogen oxides, especially nitric oxide (NO) (15, 16), which has bactericidal properties and inhibits and/or destroys a wide range of microorganisms (17).
Studies have demonstrated that the concentration of NO in caries free children is more than 50 μm, while this value was reported as less than 50 μm in children with ECC and less than 35 µm in children with severe ECC (18). In addition, it was pointed out that increasing NO concentration can play a defensive role against caries (19).
2. Objectives
Therefore, the current study aimed to systematically review and meta-analyze the relationship between dental caries in children and the concentration of salivary nitric oxide. In this regard, the effect of confounders, if any, can be explained by the performance of a meta-analysis.
3. Methods
3.1. The Inclusion and Exclusion Criteria
These criteria were determined based on patient, intervention, comparison, outcome (PICO). In the present study, P signifies children with dental caries which did not have any notable medical conditions, I which represents “Intervention” does not apply since the study was observational, C included caries-free children who were regarded as the control group in the initial studies, and O includes DMFT (decayed-missing-filled teeth for permanent teeth) and dmft (decayed-missing-filled teeth for primary teeth) indices in the group of children with caries and the control group. The current research included case-control and cross-sectional studies. Moreover, all the published studies from any time until the end of January 2020 have been among the included series. The search was carried out on March 27, 2020, and the documents are published in English and Persian.
3.2. Search Strategy
PubMed, Web of Science, Scopus, Science Direct, and Iranian Magiran and SID databases were searched using the keywords “nitric oxide”, “salivary”, “caries”, “DMFT Index”, “children”, “Early childhood caries” and OR, AND and NOT operators.
The search strategy in the PubMed database was as follows: The list of published documentary sources was reviewed to select more studies and increase the sensitivity of the search, and EndNote software was used to manage the resources.
3.3. Study Selection
Initially, duplicate documents were excluded due to overlapping content, and screening was performed based on the title and abstract of the primary studies. The unrelated articles were then removed from the study, and to match with inclusion and exclusion criteria, full-text articles were extracted by two reviewers and the contradictions were discussed.
3.4. Data Extraction
Data extraction was performed by two independent reviewers. The extracted variables included the first author’s last name, article title, journal name, year of publication, place of study, the sample size in the group of children with dental caries, sample size in the control group, method of selection of cases and controls, case-control matching based on age and gender, the mean and standard deviation of DMFT, dmft, and salivary nitric oxide (NO) concentration, as well as the evaluation method of these indices in the case and control groups. Moreover, in studies that reported only nitrite and nitrate concentrations, nitric oxide concentration was calculated by the sum of nitrate and nitrite concentrations (Griess Reaction method) and extracted from articles (20).
3.5. Quality Assessment
Preliminary studies were assessed by two independent reviewers using the Newcastle-Ottawa scale (NOS) checklist which gives a score between 0 and 9 and were eligible for the study (21). Thereafter, the articles with a score of less than 5 were excluded from the study.
3.6. Statistical Methods
The obtained data were analyzed in Stata software (version 11). The heterogeneity index of the studies was determined by I2 test and the random effect model was used to estimate the standardized difference of the mean salivary NO concentration in the group of children with caries, as compared to the control group. The inverse variance method and Cohen’s kappa statistic were also used for the estimation. Subsequently, the point estimate of the standardized difference of mean salivary NO concentration was calculated in the forest plots at 95% confidence interval. In this diagram, the size of the square indicates the weight of each study and the lines on either side show a 95% confidence interval. In cases where the confidence interval is not zero, the observed difference is statistically significant. In addition, the impact of each initial study on the overall estimate was assessed by sensitivity analysis.
4. Results
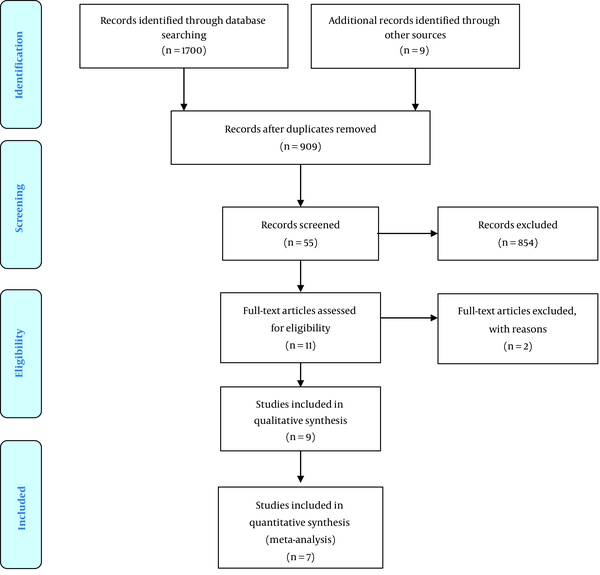
A total number of 1709 articles were identified after searching various databases and based on the strategy mentioned in the method section. Thereafter, due to overlapping content, duplicate documents were excluded and 55 related studies were identified based on the title and abstract. Subsequently, the full-text of 11 articles were evaluated out of which 2 articles (1 article due to poor study quality (22) and 1 article due to incorrect NO concentration measurement unit (20)) were ruled out from the study. Finally, the quality assessment was performed on nine articles, all of which had the minimum score required to enter the study. Out of these studies, two articles (23, 24) were only evaluated in the systematic review due to the lack of standard deviation and in total, the evidence from seven studies entered the meta-analysis (Figure 1).
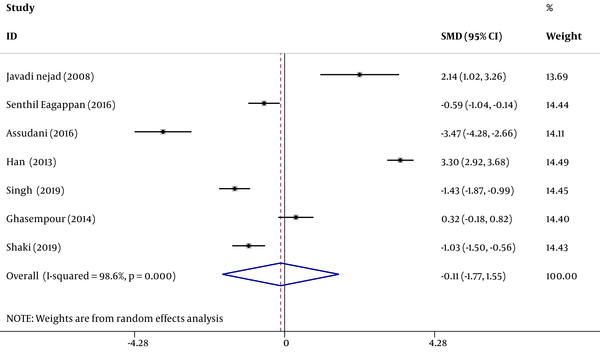
Seven pieces of evidence were extracted from seven studies (14, 18, 19, 25-28) in which the mean salivary NO concentration in children with caries was compared with the control group (Table 1).
| First Author, Publication Year | Area of Study | Type of Study | Control (Without Caries)-NO Concentration | Case (with Caries)-NO Concentration | Score of Quality Assessment | ||
|---|---|---|---|---|---|---|---|
| Sample Size | Mean ± SD | Sample Size | Mean ± SD | ||||
| Javadinejad et al., 2008 (25) | Iran | Case-control | 10 | 38.1 ± 7.29 | 10 | 51 ± 4.42 | 7 |
| Han et al., 2013 (14) | Korea | Cross-sectional | 133 | 58.94 ± 15.79 | 124 | 109.75 ± 14.96 | 7 |
| Ghasempour et al., 2014 (26) | Iran | Case-control | 31 | 70.07 ± 53.66 | 31 | 88.14 ± 58.77 | 6 |
| Senthil Eagappan et al., 2016 (18) | India | Case-control | 40 | 51.2 ± 8.3457 | 40 | 47.1 ± 5.2614 | 7 |
| Assudani et al., 2016 (19) | Belagavi | Case-control | 30 | 443.2 ± 130.07 | 30 | 110.8 ± 37.86 | 6 |
| Singh and Singh, 2019 (27) | India | Case-control | 50 | 54.07 ± 15.68 | 50 | 34.84 ± 10.79 | 6 |
| Shaki et al., 2019 (28) | Iran | Cross-sectional | 40 | 33.33 ± 20.8 | 40 | 16.04 ± 11.5 | 7 |
Charactristics of Primary Studies Included in Meta-Analysis
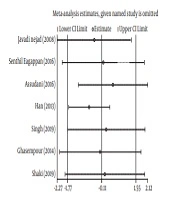
In these seven studies, the mean salivary NO concentration in children with dental caries was compared with that in the control group. In three studies (14, 25, 26) the mean of this index in children with caries was higher, as compared to the control group and the observed differences were statistically significant in two of these studies (14, 25). In other words, in four studies, the mean salivary NO concentration in children with dental caries was lower than that in the control group and the observed differences were statistically significant in all four studies (18, 19, 27, 28). The results of heterogeneous indicators showed that the discrepancy between the initial studies was statistically significant (I2 = 98.6%, Q = 442.03, P = 0.000). Combining the results of preliminary studies based on the random effect model demonstrated that the mean standardized difference is -0.11 (CI 95%: -1.77, 1.55) (Figure 2). Furthermore, as illustrated by the results of sensitivity analysis, the effect of each of the initial studies on the overall estimate was not significant (Figure 3).
5. Discussion
In the present study, the results of preliminary studies were combined by meta-analysis to investigate the relationship between mean salivary NO concentration and dental caries. The obtained results revealed that salivary NO index in children with dental caries was 0.11 lower than that in the control group, and this relationship is not statistically significant.
In the same vein, Rezvi and Mathew (24) indicated that salivary NO concentration was not associated with children’s oral and dental health. On the contrary, a study conducted by Carossa et al. (29) showed that the increase in dental plaque is associated with increased NO concentration indicating the host immune response to bacterial growth. Han et al. (14) found an inverse correlation between salivary NO levels and the number of salivary lactobacilli, and concluded that high salivary NO concentrations could be a protective factor against lactobacilli species. In addition, some other studies pointed to the positive correlation of plaque NO levels, DMFT, and Streptococcus mutans indicating that NO concentrations can be used as a screening tool to predict the rate of dental caries (30). Bayindir et al. (31) also believed that plaque NO concentration was higher in adults with higher DMFT.
Saliva can affect the prevalence of dental caries in four general ways: (1) the flushing effect which reduces plaque accumulation; (2) reduction of enamel solubility by means of calcium, phosphate, and fluoride; (3) buffering and neutralizing the acids produced by cariogenic organisms; and (4) antibacterial activity (18). Saliva plays a peculiar role among all internal defense factors (including dental morphology, general health, and nutritional status) and external factors (e.g., microbial flora, oral-dental health conditions, and fluoride use). Due to the high sensitivity and progress in measurement methods, there is a promising future for salivary biomarkers (32). Most of the compounds in the blood are also found in saliva; accordingly, saliva is functionally equivalent to serum reflecting the physiological state of the body, including hormonal, emotional, nutritional, and metabolic changes. Therefore, saliva is often used in cases where it is predicted that body fluids will be frequently sampled, blood sampling will not be morally confirmed, or both (33). It is difficult to evaluate each type of anti-caries factor as a single unit, since saliva can change all compounds in different ways (34). Furthermore, antimicrobial proteins can influence each other and weaken or reinforce each other’s effects (25) and no antibacterial compound alone is sufficient to determine the likelihood of dental caries (35).
In general, saliva contains several microorganisms that produce nitrate, including Veillonella Species, Nocardia Species, Staphylococcus epidermidis, Staphylococcus aureus, and Corynebacterium pseudodiptheriticum (18). In hypoxic conditions, these facultative anaerobes use nitrate as an electron receptor instead of oxygen to produce adenosine triphosphate (ATP) and energy for the oxidation of carbon component and they produce nitrate reductase enzyme. Therefore, nitrate is converted to nitrite during this process and its concentration is reduced in saliva. Nitrite is a waste product for bacteria, while it has antimicrobial properties for humans (36). The exposure of the produced nitrite to the acidic environment adjacent to the tooth leads to formation of a complex of nitrous oxide (N2O) and nitrous acid (HNO2) which is unstable and decomposes into nitric oxide (NO) and Nitrogen dioxide (NO2) (22).
Nitric oxide is a signaling molecule that can affect many physiological and pathological processes. It serves as an active radical in the non-specific defense mechanism in the oral cavity (37). NO enters the saliva through capillary endothelium, nerve fibers, and macrophages, and nitric oxide can also be synthesized in acinar cells of salivary glands (38). It can easily penetrate the cell membrane and destroy microorganisms by the inhibition of iron-containing DNA synthases, reaction with the iron-sulfur center of mitochondrial enzymes in the respiratory chain, or combination with superoxide and changing to highly reactive hydroxyl radical (39). Therefore, it is believed that NO can exert its antibacterial properties in two ways: (1) preventing bacterial growth and (2) Increased cytotoxicity by salivary macrophages (37). Leukocytes of the oral cavity consist of 90% of monoclonal leukocytes (PMNs and neutrophils) and 10% of mononuclear cells; 80% of neutrophils in the oral cavity are viable and functional. Neutrophils also play a key role in the protection of the host against microbes and inflammatory pathogens (40). They migrate into the mucous membranes of the gastrointestinal and respiratory tracts. In the absence of stimuli, they spontaneously synthesize radical superoxide, hypochlorite, and NO in the oral environment by the NO synthases enzyme. Oral neutrophils are activated by different ligands, one of which is the cell walls of Gram-negative bacteria (such as LPS) which can lead to the production of Tumor necrosis factor-alpha (TNF-α) (41).
Based on a study conducted by Doel et al. (34), people with higher salivary nitrate and nitrites experience fewer caries, compared to the control group. Studies have also shown that the conversion of nitrite takes place in the acidic environment of the plaque (pH less than 7), and plaque deposition results in the production of NO synthases, which in turn, leads to the breakdown of L-arginine and the production of NO. Accordingly, poor oral hygiene produces large amounts of NO; however, high values of NO cannot prevent carious properties of dental plaque. Therefore, it is believed that increased plaque thickness and caries activity may cause this discrepancy (25). By swallowing saliva, stomach acid decomposes nitrite into NO and other nitrogen oxides and acts as a defense system against gastrointestinal pathogens, a regulator of platelet activity, gastrointestinal tract motility, and microcirculation (42). The items mentioned above are summarized in Figure 4.
It has been hypothesized that increased nitrate uptake in children with dental caries can protect the tooth against decay by preventing bacterial growth (22). The studies have demonstrated that plasma nitrate concentration does not change before and after the consumption of foods containing nitrate (43). Recently, there have been concerns over the presence of nitrate in food and its harmful effects on human health. However, epidemiological studies have not yet been able to prove this hypothesis (22). Apart from the nitrate present in food, it can also be synthesized in the human body through some interactions. The synthesis of endogenous nitrate varies regardless of the amount of nitrate received from food (44). Akgul et al. (45), showed that increasing NO concentration in multiple assessments after composite resin restorations could be associated with the release of residual monomers and bioactive substances into the saliva. In the mentioned study, the maximum monomer released was observed seven days after the restoration insertion. Therefore, monomers released from resin-based filling materials lead to the production of reactive oxygen species and affect intracellular redox balance (46); moreover, it can increase bacterial growth (45). Another study showed that NO levels were significantly reduced by mobile and wireless internet (Wi-Fi) electromagnetic waves; however, no study has confirmed the effect of these waves on salivary NO levels (37).
In a study performed by Subramaniam et al. (47) on NO concentration in children with Down syndrome, an inverse relationship was detected between the concentration of NO and dental caries in permanent teeth in normal children. NO concentration was reported more frequently in children with Down syndrome. Salivary NO showed no relationship with dental caries and oral health in children with Down syndrome (47). However, several factors increase the incidence of dental caries in children with Down syndrome, such as delayed tooth growth, congenitally missing teeth, high pH values and salivary bicarbonate, microdontia, the presence of interdental spaces, and shallow dental fissures (48). Another study conducted by Subramaniam et al. (49) on children with cerebral palsy reported an inverse relationship between salivary NO levels and dental caries parameters in both groups of normal children and children with cerebral palsy.
Also, Garg et al. (50) conducted a study on the salivary NO concentration in children with congenital heart disease (CHDs) and demonstrated that the mean salivary NO concentration in children in the experimental group was much lower, as compared to the control group, and this difference was statistically significant. Low concentrations of NO in children with CHDs may be directly related to their high rate of caries. Also the mean salivary NO concentration in children with CHDs decreases with age. This can be attributed to an increase in the severity of heart disease, frequency of hospitalizations, and medicines that reduce saliva flow and contain fermented sugar. All of these factors may lead to poor dietary nitrate intake (50, 51). In Addition, dental care in children with CHDs requires special attention since these patients are more prone to infective endocarditis due to invasive dental procedures. Therefore, oral hygiene receives less attention in these patients and they are likely to have a high risk of caries due to the developmental defects of enamel (52, 53), but the role of NO in saliva and factors affecting its concentration is still open to debate (23).
5.1. Limitations
One of the limitations of this study was that the data of those studies which only reported the mean concentration of salivary nitric oxide and standard deviation were not extractable. In addition, a wrong unit was mentioned for this purpose and despite the performed correspondence, these studies lacked the requirements for meta-analysis.
We were also unable to access the EMBASE database. On the other hand, this relationship may have been insignificant due to the low number of participants in the reviewed articles. In this regard, to prove this hypothesis, it is necessary to conduct further studies in this field with more subjects. Furthermore, in these studies, the effect of confounders, such as electromagnetic waves, the number of restored teeth with metal restoration or composite resin has not been considered.
6. Conclusions
This meta-analysis study showed that salivary NO concentration was not significantly associated with dental caries, and since salivary NO concentrations can be affected by various factors, it could not be sufficient to determine the likelihood of dental caries.